A Buffer Is A Substance That
Muz Play
Mar 23, 2025 · 7 min read
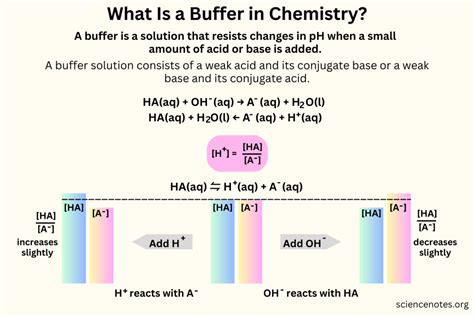
Table of Contents
A Buffer is a Substance That… Resists Change! Understanding Buffer Solutions in Chemistry
A buffer is a substance that resists changes in pH upon the addition of small amounts of acid or base. This crucial property makes buffers indispensable in various chemical and biological systems, ensuring stability and preventing drastic shifts in acidity or alkalinity. Understanding what a buffer is, how it works, and its diverse applications is crucial for anyone studying chemistry, biochemistry, or related fields. This comprehensive guide will delve into the intricacies of buffer solutions, exploring their composition, mechanism of action, and significance in different contexts.
What Makes a Buffer Solution a Buffer?
At its core, a buffer solution is an aqueous solution consisting of a weak acid and its conjugate base, or a weak base and its conjugate acid. This seemingly simple composition is the key to its remarkable ability to maintain a relatively constant pH. The weak acid/base and its conjugate pair exist in equilibrium, and this equilibrium is the cornerstone of the buffering effect.
The Equilibrium Magic: How Buffers Resist pH Changes
When a small amount of strong acid (like HCl) is added to a buffer solution, the conjugate base reacts with the added H⁺ ions, forming the weak acid. This reaction consumes the added H⁺ ions, preventing a significant decrease in pH. Conversely, when a small amount of strong base (like NaOH) is added, the weak acid in the buffer reacts with the added OH⁻ ions, forming water and the conjugate base. This neutralizes the added OH⁻, preventing a significant increase in pH.
This ability to neutralize both added acids and bases is the essence of a buffer's function. The equilibrium between the weak acid/base and its conjugate constantly shifts to counteract the changes imposed by the addition of strong acids or bases, maintaining the pH within a relatively narrow range.
Types of Buffer Solutions
Buffers aren't one-size-fits-all; different buffers are optimized for different pH ranges. The specific pH a buffer maintains is determined by the pKa (acid dissociation constant) of the weak acid component. The Henderson-Hasselbalch equation provides a quantitative relationship between pH, pKa, and the concentrations of the acid and its conjugate base:
pH = pKa + log ([conjugate base]/[acid])
This equation highlights that a buffer is most effective when the concentrations of the weak acid and its conjugate base are roughly equal, resulting in a pH close to the pKa. Several common types of buffer solutions exist, each suitable for specific applications:
1. Acetate Buffer: A Classic Choice
The acetate buffer, consisting of acetic acid (CH₃COOH) and its conjugate base, acetate (CH₃COO⁻), is a frequently used buffer in the pH range of 3.7 to 5.7. Its relatively low cost and readily available components make it a popular choice for various laboratory applications.
2. Phosphate Buffer: Versatile and Biologically Relevant
Phosphate buffers, employing different forms of phosphoric acid (H₃PO₄) and its conjugate bases, are widely used due to their versatility. They can buffer in several pH ranges depending on the specific phosphate species used, making them particularly useful in biological systems where the pH often needs to be precisely controlled.
3. Tris Buffer: A Biological Workhorse
Tris(hydroxymethyl)aminomethane (Tris) buffer is a commonly employed buffer in biochemistry and molecular biology, primarily for applications in the pH range of 7 to 9. Its relatively high buffering capacity and biocompatibility make it suitable for many biological experiments.
Calculating Buffer Capacity and pH
Understanding how to calculate the pH of a buffer solution and its buffer capacity is essential for effective utilization. The Henderson-Hasselbalch equation is vital for pH calculation, as previously discussed.
Buffer Capacity: A Measure of Effectiveness
Buffer capacity refers to the amount of strong acid or base a buffer can neutralize before a significant change in pH occurs. A higher buffer capacity indicates greater resistance to pH changes. Several factors influence buffer capacity:
- Concentration of the buffer components: Higher concentrations lead to higher buffer capacity.
- Ratio of acid to conjugate base: The buffer capacity is highest when the concentrations of the acid and conjugate base are equal (i.e., when pH = pKa).
- The nature of the weak acid/base: Different weak acids/bases have inherent differences in their buffering capabilities.
Precise calculations of buffer capacity often involve more complex equations considering the equilibrium constants and the total concentration of the buffer components.
Applications of Buffer Solutions: A Wide Spectrum
The applications of buffer solutions are extensive and span various scientific disciplines:
1. Biochemistry and Biology: Maintaining Cellular Life
Buffers are crucial for maintaining the pH within the narrow physiological ranges required for enzyme activity and other biological processes. Biological systems employ various endogenous buffer systems, such as phosphate, bicarbonate, and protein buffers, to ensure stability.
2. Analytical Chemistry: Ensuring Accurate Measurements
Buffers are essential in many analytical procedures, especially those involving titrations or pH-sensitive measurements. They provide a stable pH environment, preventing erratic results and ensuring the accuracy of experimental data.
3. Pharmaceutical and Medical Applications: Drug Delivery and Stability
Many pharmaceuticals require specific pH conditions for optimal stability and effectiveness. Buffers are often incorporated into drug formulations to maintain these conditions, ensuring consistent drug delivery and efficacy.
4. Industrial Processes: Controlling Reaction Conditions
Various industrial processes require precise pH control. Buffers are used to maintain optimal pH in reactions, enhancing efficiency, product yield, and reducing waste. Examples include electroplating, food processing, and textile manufacturing.
5. Environmental Science: Monitoring and Maintaining Aquatic Ecosystems
Maintaining the pH of aquatic environments is crucial for aquatic life. Buffers can play a role in mitigating the effects of acid rain or other pH-altering factors in lakes, rivers, and other water bodies.
Choosing the Right Buffer: Factors to Consider
Selecting the appropriate buffer for a specific application involves considering several factors:
- Desired pH range: The pKa of the weak acid should be close to the target pH.
- Buffer capacity: The buffer must have sufficient capacity to withstand the expected pH changes.
- Ionic strength: The ionic strength of the buffer can influence the activity of ions in the solution.
- Solubility and stability: The buffer components should be soluble and stable under the experimental conditions.
- Toxicity and biocompatibility: For biological applications, the buffer must be non-toxic and biocompatible.
Beyond the Basics: Advanced Buffer Concepts
The principles of buffer solutions extend to more complex scenarios and concepts:
1. Zwitterions and their Buffering Capacity
Zwitterions, molecules containing both positive and negative charges, exhibit buffering properties due to their ability to act as both acids and bases. Amino acids, the building blocks of proteins, are classic examples of zwitterions with buffering capacity.
2. Polyprotic Acids and their Multiple Buffer Regions
Polyprotic acids, like phosphoric acid, can donate more than one proton, resulting in multiple buffering regions corresponding to their different pKa values. This allows for broader pH control using a single polyprotic acid and its conjugate bases.
3. Buffering in Non-Aqueous Solvents
While most buffer systems are aqueous, buffering principles also apply to non-aqueous solvents, albeit with altered equilibrium constants and behavior.
Conclusion: The Indispensable Role of Buffers
In conclusion, a buffer is a substance that maintains a relatively constant pH despite the addition of small amounts of acid or base. This critical property stems from the equilibrium between a weak acid and its conjugate base (or a weak base and its conjugate acid). Buffers are essential in countless applications across diverse fields, from maintaining the delicate pH balance in biological systems to controlling reaction conditions in industrial processes. Understanding the principles governing buffer solutions is vital for anyone working in chemistry, biochemistry, or related areas. The selection of the appropriate buffer depends on several factors, ensuring the buffer's effective performance and suitability for the specific application. The study of buffers extends beyond simple aqueous systems, encompassing more complex scenarios like zwitterions and non-aqueous solutions, further highlighting their versatile and indispensable role in chemistry and beyond.
Latest Posts
Latest Posts
-
What Is A Power Stroke During Muscle Contraction
Mar 24, 2025
-
Enter The Assignment Of The Observed Transition Violet
Mar 24, 2025
-
Conflict Theorists View Gender Differences As
Mar 24, 2025
-
What Is The Subtraction Property Of Equality
Mar 24, 2025
-
Relation Between Electric Field And Potential
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about A Buffer Is A Substance That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
