A Disaccharide Is Formed By The Chemical Bonding Of
Muz Play
Mar 29, 2025 · 6 min read
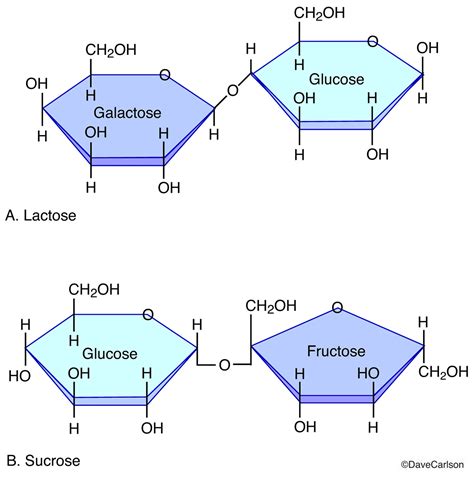
Table of Contents
A Disaccharide is Formed by the Chemical Bonding of Monosaccharides: A Deep Dive into Glycosidic Bonds
Disaccharides, the sweet taste of many foods, are fundamental carbohydrate units playing crucial roles in various biological processes. Understanding how these molecules are formed is key to understanding their function and importance in living organisms. This article delves into the fascinating world of disaccharides, exploring the chemical bonding that brings them together and the implications of their diverse structures.
Understanding the Building Blocks: Monosaccharides
Before diving into disaccharide formation, let's establish a solid understanding of their fundamental components: monosaccharides. These are the simplest form of carbohydrates, often referred to as simple sugars. Key monosaccharides include:
- Glucose: The most abundant monosaccharide, serving as the primary energy source for many organisms. It's found in fruits, honey, and corn syrup.
- Fructose: Known as fruit sugar, fructose is found naturally in fruits and honey. It's sweeter than glucose.
- Galactose: Less common than glucose and fructose, galactose is a component of lactose, the sugar found in milk.
These monosaccharides possess multiple hydroxyl (-OH) groups and a carbonyl group (C=O), either an aldehyde (–CHO) or a ketone (–C=O). This combination of functional groups is crucial for their chemical reactivity and ability to form disaccharides.
The Formation of Glycosidic Bonds: The Key to Disaccharide Formation
The chemical bonding that unites monosaccharides to form disaccharides is known as a glycosidic bond. This is a covalent bond formed between the hemiacetal or hemiketal group of one monosaccharide and the hydroxyl group of another monosaccharide.
This bond formation involves a dehydration reaction, also known as a condensation reaction. During this process, a molecule of water (H₂O) is removed, with the hydroxyl group of one monosaccharide contributing an -OH group and the hemiacetal/hemiketal group of the other contributing an -H. The remaining oxygen atom forms the bridge connecting the two monosaccharides. The resulting bond is an O-glycosidic bond.
Understanding Hemiacetals and Hemiketals:
Before the glycosidic bond can form, the monosaccharides must first undergo cyclization. The aldehyde or ketone group reacts with a hydroxyl group within the same molecule, creating a cyclic structure. This forms a ring structure containing an oxygen atom. The carbon atom that was part of the carbonyl group becomes a chiral center (anomeric carbon).
- Hemiacetals are formed when the carbonyl group is an aldehyde.
- Hemiketals are formed when the carbonyl group is a ketone.
This newly formed hemiacetal or hemiketal group is crucial for the subsequent formation of the glycosidic bond. It is the reactive site that will link to another monosaccharide.
Types of Glycosidic Bonds: α and β
The orientation of the hydroxyl group on the anomeric carbon plays a crucial role in determining the type of glycosidic bond and, consequently, the properties of the resulting disaccharide. There are two possible orientations:
- α (alpha) configuration: The hydroxyl group on the anomeric carbon is below the plane of the ring.
- β (beta) configuration: The hydroxyl group on the anomeric carbon is above the plane of the ring.
The type of glycosidic bond (α or β) significantly impacts the properties and biological function of the disaccharide. For example, α(1→4) glycosidic bonds, as found in maltose and sucrose, have different digestibility properties compared to β(1→4) glycosidic bonds, like those in lactose and cellobiose.
Common Disaccharides: A Closer Look
Several common disaccharides are formed through various combinations of monosaccharides and glycosidic linkages. Let's examine a few examples:
1. Sucrose (Table Sugar)
Sucrose is formed from the combination of:
- Glucose: α-D-glucose
- Fructose: β-D-fructose
The bond is an α(1→β2) glycosidic bond, linking the anomeric carbon of glucose to the anomeric carbon of fructose. Sucrose is a non-reducing sugar, meaning it cannot act as a reducing agent because both anomeric carbons are involved in the glycosidic bond.
2. Maltose (Malt Sugar)
Maltose is formed from two glucose units linked by an:
- α(1→4) glycosidic bond.
It's a reducing sugar, meaning the free anomeric carbon on the glucose unit not involved in the glycosidic bond can undergo oxidation-reduction reactions.
3. Lactose (Milk Sugar)
Lactose is found in milk and is composed of:
- Galactose: β-D-galactose
- Glucose: β-D-glucose
The bond is a β(1→4) glycosidic bond. Like maltose, it is a reducing sugar. Lactose intolerance stems from a deficiency in lactase, the enzyme responsible for breaking down lactose.
4. Cellobiose
Cellobiose, a disaccharide derived from cellulose, is composed of two glucose units linked by a:
- β(1→4) glycosidic bond.
Unlike maltose, the orientation of the glycosidic bond makes cellobiose less easily digestible by humans.
The Importance of Disaccharides: Biological Roles and Applications
Disaccharides play crucial roles in various biological processes and have wide-ranging applications. Some key roles and applications include:
- Energy Source: Many disaccharides, like sucrose and maltose, serve as readily available energy sources for organisms. They are hydrolyzed into their constituent monosaccharides, which can then be metabolized to produce ATP.
- Structural Components: Some disaccharides, particularly those with β-glycosidic bonds, contribute to the structure of plant cell walls (e.g., cellobiose in cellulose).
- Food Industry: Sucrose, a major disaccharide, is extensively used as a sweetener in various food products.
- Medical Applications: Certain disaccharides are used in various medical applications, including as components of intravenous solutions or as part of specialized diets.
- Biosynthesis of Polysaccharides: Disaccharides serve as precursors for the synthesis of larger polysaccharides, such as starch and glycogen.
Factors Affecting Glycosidic Bond Formation and Disaccharide Properties
Several factors influence the formation and properties of glycosidic bonds and the resulting disaccharides:
- Stereochemistry of Monosaccharides: The α or β configuration of the anomeric carbon significantly impacts the bond's properties.
- Position of Glycosidic Linkage: The position of the bond between the monosaccharides (e.g., 1→4, 1→6) influences the disaccharide's structure and properties.
- Reaction Conditions: Factors like temperature, pH, and the presence of catalysts affect the rate and efficiency of glycosidic bond formation.
- Enzyme Specificity: Enzymes involved in glycosidic bond formation and hydrolysis exhibit high specificity for substrate structures.
Hydrolysis of Disaccharides: Breaking the Bonds
Disaccharides can be broken down into their constituent monosaccharides through a process called hydrolysis. This process involves the addition of a water molecule, breaking the glycosidic bond and releasing the individual monosaccharides. This reaction is often catalyzed by enzymes known as glycosidases, which are highly specific to the type of glycosidic bond they hydrolyze.
Conclusion: A Sweet World of Chemical Bonds
The formation of a disaccharide through glycosidic bonds is a fundamental process in carbohydrate chemistry with significant biological implications. The specific type of glycosidic bond (α or β) and the position of the linkage greatly influence the properties and functions of the resulting disaccharide. From the sweet taste of table sugar to the structural components of plant cell walls, disaccharides play diverse and essential roles in living organisms, highlighting the importance of understanding these fundamental chemical bonds. Further research into glycosidic bonds continues to provide valuable insights into various biological processes and opens new avenues for applications in diverse fields. The continuing exploration of the chemistry and biology of disaccharides ensures that our knowledge of these essential molecules will continue to expand, unlocking further potentials for scientific and technological advancements.
Latest Posts
Latest Posts
-
Table Salt Is A Pure Substance
Mar 31, 2025
-
What Makes Sour Patch Kids Sour
Mar 31, 2025
-
Is Urea The Same As Uric Acid
Mar 31, 2025
-
How To Determine The Highest Boiling Point
Mar 31, 2025
-
How To Explain 10x In Lab Math
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about A Disaccharide Is Formed By The Chemical Bonding Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
