A Triple Bond Is Generally Composed Of
Muz Play
Mar 15, 2025 · 5 min read
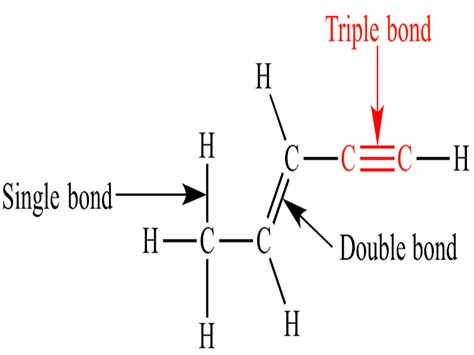
Table of Contents
A Triple Bond is Generally Composed of: A Deep Dive into Molecular Structure and Bonding
A triple bond, a cornerstone of organic and inorganic chemistry, represents the strongest type of covalent bond between two atoms. Understanding its composition is crucial for grasping the reactivity, properties, and behavior of numerous molecules. This comprehensive exploration will delve into the intricacies of triple bonds, exploring their formation, characteristics, and implications in various chemical contexts.
The Building Blocks: Sigma and Pi Bonds
A triple bond isn't a single, massively strong interaction. Instead, it's a composite of one sigma (σ) bond and two pi (π) bonds. This fundamental distinction dictates the bond's unique properties and reactivity. Let's examine each component:
The Sigma (σ) Bond: The Backbone of Strength
The sigma bond is the foundation of any covalent bond, including triple bonds. It forms through head-on overlap of atomic orbitals. This direct, end-to-end interaction creates a high electron density region concentrated along the internuclear axis – the imaginary line connecting the two bonded atoms. The sigma bond is strongest due to this effective orbital overlap, providing a robust framework for the triple bond. In triple bonds involving carbon, the sigma bond usually originates from the hybridization of atomic orbitals, often sp hybridization.
The Pi (π) Bonds: Adding Stability and Reactivity
Pi bonds, the additional components of a triple bond, arise from the sideways overlap of p orbitals. Unlike the sigma bond's axial overlap, pi bonds exhibit electron density above and below the internuclear axis. This configuration results in a less effective overlap compared to sigma bonds, making pi bonds generally weaker than sigma bonds. However, their presence significantly strengthens the overall triple bond and impacts its reactivity. Crucially, the two pi bonds in a triple bond are perpendicular to each other and to the sigma bond, creating a specific spatial arrangement that influences molecular geometry and reactivity.
Formation of a Triple Bond: Atomic Orbital Hybridization
The formation of a triple bond is intricately linked to the hybridization of atomic orbitals. Hybridization is a theoretical concept that describes the mixing of atomic orbitals within an atom to form new hybrid orbitals with different shapes and energies. This process is particularly significant in the formation of triple bonds, especially those involving carbon atoms.
The Role of sp Hybridization
In molecules containing triple bonds, such as acetylene (C₂H₂), the carbon atoms undergo sp hybridization. This process involves the mixing of one s orbital and one p orbital from each carbon atom, resulting in two sp hybrid orbitals. These hybrid orbitals are oriented at 180 degrees to each other, leading to a linear molecular geometry. The remaining two p orbitals on each carbon atom remain unhybridized and participate in the formation of the two pi bonds.
The sp hybridized orbitals from each carbon atom overlap head-on to form the sigma bond. Subsequently, the unhybridized p orbitals from each carbon atom overlap sideways to form the two pi bonds, completing the triple bond. The resulting molecule is characterized by the strong triple bond and its linear geometry.
Examples Beyond Carbon: Nitrogen's Triple Bond
While carbon is commonly associated with triple bonds, other elements also participate in this type of strong bonding. A notable example is nitrogen gas (N₂), where two nitrogen atoms are connected by a triple bond. Similar to carbon, nitrogen atoms undergo hybridization (though the specifics differ slightly), creating a sigma bond and two pi bonds, leading to the remarkable stability and inertness of N₂.
Properties and Characteristics of Triple Bonds
The unique composition of triple bonds, involving both sigma and pi bonds, bestows several distinctive properties onto molecules containing them:
-
High Bond Energy: Triple bonds possess significantly higher bond energy than single or double bonds due to the combined strength of the sigma and two pi bonds. This results in strong bonds that are difficult to break.
-
Short Bond Length: The strong attraction between the atoms involved in a triple bond leads to shorter bond lengths compared to single or double bonds. This close proximity between atoms also influences the reactivity and properties of the molecule.
-
Linear Geometry (for sp hybridized atoms): As discussed earlier, sp hybridization results in linear geometry around the atoms involved in the triple bond. This geometric feature has implications for the molecule's overall shape and reactivity.
-
Reactivity: While strong, triple bonds are not entirely unreactive. The pi bonds, being less stable than the sigma bond, are more susceptible to attack by electrophilic or nucleophilic reagents. This reactivity plays a crucial role in various organic reactions, including additions and reductions.
-
Acidity: Molecules with triple bonds can exhibit acidic behavior, particularly when the triple bond is adjacent to an electron-withdrawing group. This acidity is often influenced by the electron density around the triple bond.
Implications and Applications of Triple Bonds
Triple bonds play a vital role in various areas of chemistry and beyond:
Organic Chemistry: Alkynes and their Reactions
Alkynes, hydrocarbons containing at least one carbon-carbon triple bond, represent a key functional group in organic chemistry. Their reactivity, particularly the addition of reagents to the pi bonds, is widely exploited in organic synthesis for the creation of more complex molecules. The linear geometry and acidity of alkynes also significantly influence their reaction pathways.
Inorganic Chemistry: Nitrogen Gas and its Importance
The nitrogen-nitrogen triple bond in N₂ is essential for life on Earth. The extraordinary stability of this bond explains nitrogen gas's inertness, its abundance in the atmosphere, and the need for specialized biological processes (nitrogen fixation) to convert it into usable forms for living organisms.
Materials Science: Carbon Nanotubes and Graphene
Carbon-carbon triple bonds are integral to the structure of carbon nanotubes and graphene. These materials exhibit exceptional mechanical strength, electrical conductivity, and other unique properties, leading to their application in advanced materials science and nanotechnology.
Conclusion: A Powerful Force in Molecular Structure
The triple bond, composed of one strong sigma bond and two weaker pi bonds, is a powerful and influential feature in molecular structure and reactivity. Its presence impacts bond energy, bond length, molecular geometry, and chemical behavior across a wide range of molecules and materials. Understanding the intricacies of triple bond formation and its characteristics is vital for advancements in organic chemistry, inorganic chemistry, materials science, and beyond. Further exploration into the nuances of triple bond interactions will undoubtedly uncover even more profound applications of this fundamental chemical entity.
Latest Posts
Latest Posts
-
Critical Thinking Vs Clinical Judgement In Nursing
May 09, 2025
-
Which Ion Has A Charge Of 2
May 09, 2025
-
What Type Of Symmetry Does A Mollusk Have
May 09, 2025
-
Is Freezing Point Intensive Or Extensive
May 09, 2025
-
The Chemical Formula Of An Ionic Compound Represents
May 09, 2025
Related Post
Thank you for visiting our website which covers about A Triple Bond Is Generally Composed Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.