According To The Kinetic Theory Of Gases
Muz Play
Mar 17, 2025 · 6 min read
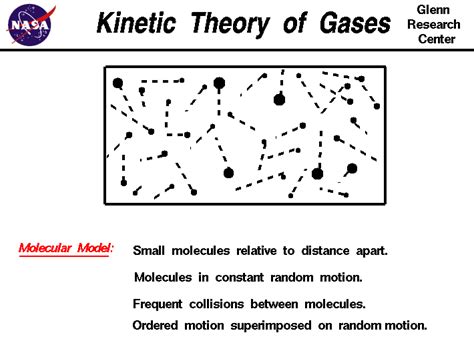
Table of Contents
According to the Kinetic Theory of Gases: A Deep Dive into Molecular Motion
The kinetic theory of gases provides a powerful framework for understanding the macroscopic properties of gases—like pressure, temperature, and volume—by considering the microscopic behavior of their constituent particles. This theory, based on a series of postulates, allows us to explain and predict gas behavior with remarkable accuracy, especially under conditions of low pressure and high temperature where intermolecular forces are relatively weak. Let's delve into the core principles, implications, and limitations of this fundamental concept in physical chemistry.
The Postulates of the Kinetic Theory of Gases
The kinetic theory rests upon several fundamental assumptions or postulates:
1. Gases Consist of Tiny Particles in Constant, Random Motion:
Gases are composed of a vast number of extremely small particles (atoms or molecules) that are in constant, chaotic motion. This ceaseless movement is responsible for many of the observed properties of gases. These particles are far apart relative to their size, leading to large interparticle distances compared to the size of the particles themselves. This is why gases are compressible.
2. The Volume of Gas Particles is Negligible Compared to the Total Volume:
The volume occupied by the gas particles themselves is considered insignificant compared to the total volume of the container holding the gas. This is a valid approximation at low pressures where the particles are widely dispersed. At high pressures, however, the volume of the particles becomes a more significant fraction of the total volume, and deviations from ideal gas behavior become more pronounced.
3. Collisions Between Gas Particles and Container Walls are Elastic:
Collisions between gas particles and the walls of the container, as well as collisions between the particles themselves, are perfectly elastic. This means that no kinetic energy is lost during these collisions. The total kinetic energy of the system remains constant in the absence of external forces.
4. The Forces of Attraction or Repulsion Between Gas Particles are Negligible:
The attractive or repulsive forces between gas particles are negligible, except during brief collisions. This assumption simplifies the analysis considerably. Real gases, however, do experience intermolecular forces (like van der Waals forces), which become more significant at lower temperatures and higher pressures.
5. The Average Kinetic Energy of Gas Particles is Proportional to Absolute Temperature:
The average kinetic energy of the gas particles is directly proportional to the absolute temperature (Kelvin scale) of the gas. This means that as the temperature increases, the average speed of the gas particles increases. This relationship is a cornerstone of the kinetic theory and provides a direct link between the microscopic world of molecular motion and the macroscopic property of temperature.
Deriving Macroscopic Properties from Microscopic Behavior
The postulates of the kinetic theory allow us to derive expressions for various macroscopic properties of gases, including:
Pressure:
Pressure is explained as the result of countless collisions between gas particles and the walls of the container. Each collision exerts a tiny force, and the cumulative effect of these collisions over time and across the container's surface produces the macroscopic pressure. The kinetic theory provides a quantitative relationship between pressure (P), volume (V), number of particles (n), and temperature (T), leading to the ideal gas law:
PV = nRT
Where R is the ideal gas constant.
Temperature:
Temperature is directly linked to the average kinetic energy of the gas particles. A higher temperature means a higher average kinetic energy and therefore faster particle speeds. This connection is crucial in understanding the relationship between temperature and gas behavior. The root-mean-square (rms) speed of gas particles is given by:
v<sub>rms</sub> = √(3RT/M)
Where M is the molar mass of the gas.
Diffusion and Effusion:
The kinetic theory also explains the processes of diffusion (the spread of gas particles throughout a given volume) and effusion (the escape of gas particles through a small hole). Graham's law of effusion, derived from the kinetic theory, states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass. Lighter gases effuse faster than heavier gases.
Deviations from Ideal Gas Behavior: Real Gases
While the ideal gas law provides a good approximation for many gases under normal conditions, real gases deviate from ideal behavior at high pressures and low temperatures. These deviations are caused by:
Intermolecular Forces:
Real gas particles experience attractive forces (e.g., van der Waals forces) that are not accounted for in the ideal gas model. These forces reduce the effective pressure exerted by the gas.
Finite Particle Volume:
The volume occupied by real gas particles is not negligible at high pressures. This reduces the available volume for the particles to move around in, leading to increased pressure.
The van der Waals Equation:
To account for these deviations, modified equations of state, such as the van der Waals equation, have been developed. The van der Waals equation incorporates correction terms for intermolecular forces (represented by 'a') and particle volume ('b'):
(P + a(n/V)²)(V - nb) = nRT
The 'a' and 'b' parameters are specific to each gas and reflect the strength of intermolecular forces and the size of the gas particles, respectively.
Applications of the Kinetic Theory of Gases
The kinetic theory of gases has numerous applications in various fields, including:
- Meteorology: Understanding atmospheric behavior, including pressure changes and wind patterns.
- Chemistry: Predicting reaction rates and equilibrium constants.
- Engineering: Designing and optimizing industrial processes involving gases, such as combustion and gas separation.
- Physics: Studying the properties of plasmas and other exotic states of matter.
Limitations of the Kinetic Theory of Gases
Despite its wide applicability, the kinetic theory has limitations:
- Ideal Gas Assumption: The theory relies heavily on the ideal gas assumptions, which are not always valid for real gases, especially at high pressures and low temperatures.
- Simplified Model: It is a simplified model that neglects many complex factors, such as the rotation and vibration of molecules and quantum mechanical effects.
- Predictive Power at Extreme Conditions: Its predictive power diminishes at very high pressures and extremely low temperatures where quantum effects become significant and intermolecular forces dominate.
Conclusion
The kinetic theory of gases provides a fundamental and insightful understanding of the macroscopic behavior of gases based on the microscopic motion of their constituent particles. While its assumptions simplify reality, the theory accurately predicts the behavior of many gases under many conditions. The deviations from ideality, however, highlight the importance of considering intermolecular forces and finite particle volume in modeling real gases accurately. The theory's elegance and power continue to be essential in various scientific and engineering disciplines. Understanding the kinetic theory is crucial for anyone wanting to grasp the underlying principles governing the behavior of gases and their diverse applications in the world around us. Future research and refinements of the kinetic theory will undoubtedly continue to enhance our understanding of matter at both the macroscopic and microscopic levels.
Latest Posts
Latest Posts
-
Name A Structural Difference Between Triglycerides And Phospholipids
Mar 17, 2025
-
Ending Materials In A Chemical Reaction
Mar 17, 2025
-
Get Energy By Eating Other Organisms
Mar 17, 2025
-
What Is A Power Function In Math
Mar 17, 2025
-
How Many Phosphate Groups Does Atp Have
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about According To The Kinetic Theory Of Gases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
