Acids And Bases Cannot Mix Together
Muz Play
Mar 15, 2025 · 6 min read
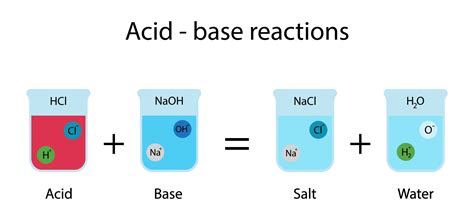
Table of Contents
Acids and Bases: Why Mixing Them is a Recipe for Reaction (and Sometimes, Disaster)
Acids and bases are fundamental chemical concepts that permeate our daily lives, from the citric acid in our oranges to the sodium hydroxide used in soap making. While seemingly disparate, they are intimately related through a fundamental principle: they react with each other. This reaction, known as neutralization, is far from benign and understanding its nuances is crucial for safety and a deeper understanding of chemistry. The statement "acids and bases cannot mix together" is fundamentally incorrect; they always react when mixed, although the extent and nature of that reaction vary dramatically. This article explores this interaction in detail, delving into the mechanisms, consequences, and practical applications of acid-base neutralization.
Understanding Acids and Bases: A Quick Refresher
Before exploring the consequences of mixing acids and bases, let's briefly review their definitions. There are several ways to define acids and bases, each offering a slightly different perspective. The most common are the Arrhenius, Brønsted-Lowry, and Lewis definitions.
Arrhenius Definition:
The simplest definition, proposed by Svante Arrhenius, defines acids as substances that produce hydrogen ions (H⁺) when dissolved in water, and bases as substances that produce hydroxide ions (OH⁻) in water. This definition is helpful for understanding simple acid-base reactions, but it's limited in its scope.
Brønsted-Lowry Definition:
A more encompassing definition, the Brønsted-Lowry theory, defines acids as proton donors and bases as proton acceptors. This definition extends beyond aqueous solutions and allows for a wider range of substances to be classified as acids or bases. For instance, ammonia (NH₃) can act as a base by accepting a proton, even though it doesn't produce hydroxide ions directly in water.
Lewis Definition:
The most general definition, the Lewis theory, defines acids as electron pair acceptors and bases as electron pair donors. This broad definition encompasses a vast array of reactions, going beyond the simple proton transfer seen in Brønsted-Lowry theory. Many reactions that aren't traditionally considered acid-base reactions under the other definitions fall under the Lewis definition.
The Inevitable Reaction: Neutralization
When an acid and a base are mixed, they undergo a neutralization reaction. This reaction essentially involves the combination of hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base to form water (H₂O). The other product is a salt, an ionic compound formed from the cation of the base and the anion of the acid.
General Equation: Acid + Base → Salt + Water
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Here, hydrochloric acid donates a proton (H⁺) to the hydroxide ion (OH⁻) from sodium hydroxide, forming water. The remaining ions, sodium (Na⁺) and chloride (Cl⁻), combine to form sodium chloride (NaCl), common table salt.
The Extent of the Reaction: Strong vs. Weak Acids and Bases
The strength of an acid or base significantly influences the extent of the neutralization reaction. Strong acids and bases completely dissociate into ions in water, meaning all their H⁺ or OH⁻ ions are available to react. Weak acids and bases only partially dissociate, meaning a significant portion of the acid or base remains in its undissociated form.
The neutralization reaction between a strong acid and a strong base is essentially complete, meaning almost all the H⁺ and OH⁻ ions react to form water. However, the reaction between a weak acid and a weak base is less complete, reaching an equilibrium where some undissociated acid and base remain in the solution.
Consequences of Mixing Acids and Bases: Beyond Neutralization
The consequences of mixing acids and bases extend far beyond the simple formation of salt and water. The reaction can be:
Exothermic:
Neutralization reactions are typically exothermic, meaning they release heat. The amount of heat released depends on the strength and concentration of the acid and base. Mixing strong, concentrated acids and bases can generate significant amounts of heat, potentially leading to boiling or even explosions if not handled carefully.
pH Change:
The most significant consequence is the change in pH. The pH scale measures the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is neutral, below 7 is acidic, and above 7 is basic. Mixing an acid and a base aims to achieve a neutral pH of 7; however, the precise pH depends on the relative strengths and concentrations of the reactants. A careful stoichiometric calculation is needed to determine the final pH.
Formation of Precipitates:
In some cases, the salt formed during neutralization may be insoluble in water. This leads to the formation of a precipitate, a solid that separates from the solution. The characteristics of the precipitate, such as its color and solubility, can be useful in identifying the reactants.
Gas Evolution:
Certain acid-base reactions can produce gases. For example, the reaction between an acid and a carbonate or bicarbonate will produce carbon dioxide gas. This gas evolution can be a safety hazard if not properly managed, especially in closed containers.
Practical Applications: Exploiting the Power of Neutralization
The neutralization reaction has numerous practical applications:
Acid Rain Remediation:
Limestone, a naturally occurring base, is often used to neutralize acidic soil and lakes affected by acid rain. The reaction between limestone (calcium carbonate) and the acidic components in rainwater helps to restore the pH to a more neutral level.
Antacid Tablets:
Antacids are commonly used to relieve heartburn, which is caused by excess stomach acid (hydrochloric acid). Antacids contain bases, such as calcium carbonate or magnesium hydroxide, which neutralize the excess acid, relieving discomfort.
Wastewater Treatment:
Neutralization is a crucial step in wastewater treatment. Industrial wastewater often contains acidic or basic components that need to be neutralized before discharge to protect aquatic ecosystems.
Chemical Synthesis:
Neutralization reactions play a critical role in chemical synthesis, where precise pH control is often necessary for specific reactions. By carefully controlling the addition of an acid or base, chemists can achieve the desired pH and facilitate the formation of the desired product.
Safety Precautions: Handling Acids and Bases Responsibly
Because mixing acids and bases can lead to exothermic reactions, gas evolution, and potential hazards, proper safety precautions are essential.
- Always wear appropriate personal protective equipment (PPE): This includes safety goggles, gloves, and lab coats.
- Work in a well-ventilated area: To avoid inhaling any harmful fumes.
- Add acid to base slowly and carefully: To control the reaction and prevent splashing. Never add water to a concentrated acid.
- Use appropriate glassware: Heat-resistant glassware should be used when dealing with exothermic reactions.
- Neutralize spills immediately: Follow proper spill procedures to minimize environmental and personal harm.
Conclusion: A Deeper Understanding of Acid-Base Chemistry
The assertion that acids and bases cannot mix together is a misconception. The reality is that they always react, undergoing a neutralization reaction that produces salt and water. The specifics of this reaction – the extent, the heat generated, the formation of precipitates or gases – depend on the strengths and concentrations of the reacting acid and base. Understanding these intricacies is crucial for safely handling acids and bases and harnessing their reactivity for various practical applications. From environmental remediation to chemical synthesis, mastering the principles of acid-base chemistry is essential in various scientific and industrial fields. By adhering to safety guidelines and employing careful techniques, we can utilize the power of neutralization for beneficial purposes while mitigating potential risks.
Latest Posts
Latest Posts
-
Compare And Contrast Spermatogenesis And Oogenesis In Human Cells
May 09, 2025
-
Melting Point Of Water In Kelvin Scale
May 09, 2025
-
Why Are There No Chloroplasts In Onion Cells
May 09, 2025
-
What Countries Are In Stage 2 Of Rostows Model
May 09, 2025
-
A Profit Maximizing Monopoly Will Charge A Price Of
May 09, 2025
Related Post
Thank you for visiting our website which covers about Acids And Bases Cannot Mix Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.