An Example Of Extensive Property Of Matter Is
Muz Play
Mar 17, 2025 · 6 min read
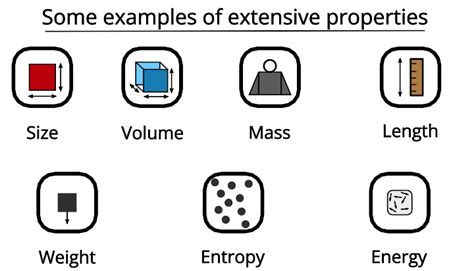
Table of Contents
An Example of the Extensive Property of Matter is... Volume!
Matter, the physical substance that makes up everything in the universe, exhibits a variety of properties. These properties can be broadly categorized as either extensive or intensive. While intensive properties, like density and temperature, remain constant regardless of the amount of matter present, extensive properties change proportionally with the amount of matter. This article will delve deep into the concept of extensive properties, using volume as a prime example, and exploring its implications across various scientific disciplines.
Understanding Extensive Properties: A Deep Dive
Extensive properties are fundamentally dependent on the size or amount of the matter being considered. Imagine a single drop of water compared to a liter of water. Both are water, sharing the same intensive properties (density, boiling point, etc.), but their extensive properties, like volume and mass, differ dramatically. The more matter you have, the larger the value of the extensive property. This simple relationship is crucial in understanding various phenomena across chemistry, physics, and engineering.
Other examples of extensive properties include:
- Mass: The amount of matter contained in an object. A larger object naturally has a greater mass.
- Length: The measurement of an object from end to end. A longer object has a greater length.
- Weight: The force exerted on an object due to gravity. A heavier object has a greater weight (although weight is technically dependent on gravitational force, it remains proportionally linked to mass).
- Energy: The capacity to do work. A larger system or a more massive object typically possesses more energy.
- Heat capacity: The amount of heat required to raise the temperature of a substance by a certain degree. A larger quantity of a substance requires more heat to change its temperature.
These properties all scale with the size of the system, a defining characteristic of extensive properties. They are additive; the total extensive property of a combined system is simply the sum of the individual properties of its constituent parts.
Volume: The Perfect Extensive Property Example
Let's focus on volume, a quintessential extensive property. Volume refers to the amount of three-dimensional space occupied by a substance or object. It's directly proportional to the amount of matter present. Double the amount of matter (under constant conditions), and you'll likely double the volume.
Understanding Volume in Different States of Matter
The concept of volume applies to all states of matter:
- Solids: The volume of a solid is generally determined by its shape and dimensions. For regular shapes like cubes or spheres, volume can be calculated using standard geometrical formulas. Irregular solids require techniques like water displacement to measure their volume.
- Liquids: Liquids conform to the shape of their container, but maintain a relatively constant volume. Measuring the volume of a liquid is straightforward using graduated cylinders, beakers, or volumetric flasks.
- Gases: Gases fill their containers completely, making their volume equal to the volume of the container. Gas volume is highly sensitive to temperature and pressure changes, a principle explored in the ideal gas law.
Calculating Volume: A Practical Approach
Calculating volume depends on the shape and state of the matter.
-
Regular solids: Simple geometric formulas apply. For example:
- Cube: Volume = side³
- Sphere: Volume = (4/3)πr³
- Cylinder: Volume = πr²h
-
Irregular solids: Water displacement is a common technique. The object is submerged in a known volume of water, and the increase in water level indicates the object's volume.
-
Liquids: Direct measurement using calibrated instruments like graduated cylinders or volumetric flasks.
-
Gases: Using the ideal gas law (PV = nRT), which relates pressure (P), volume (V), number of moles (n), ideal gas constant (R), and temperature (T).
The Significance of Volume in Various Fields
The extensive property of volume plays a crucial role across numerous scientific and engineering disciplines:
Chemistry:
- Stoichiometry: Volume (especially of gases) is vital in calculating reactant and product quantities in chemical reactions. The molar volume of gases at standard temperature and pressure (STP) is a fundamental concept in this area.
- Solution preparation: Preparing solutions of specific concentrations requires precise volume measurements. Volumetric analysis relies heavily on accurate volume determinations.
- Density calculations: Density, an intensive property, is defined as mass per unit volume. Knowing both mass and volume is necessary to calculate density.
Physics:
- Fluid mechanics: Understanding the volume of fluids (liquids and gases) is crucial for analyzing their flow, pressure, and buoyancy.
- Thermodynamics: Volume changes are central to many thermodynamic processes, particularly in the context of work done by or on a system.
- Optics: The volume of materials plays a role in their optical properties, like refractive index.
Engineering:
- Civil engineering: Volume calculations are crucial for estimating the amount of materials needed for construction projects (concrete, soil, etc.).
- Mechanical engineering: Understanding fluid volumes is essential for designing and optimizing systems involving pumps, pipes, and reservoirs.
- Chemical engineering: Volume is critical in designing and sizing reactors, storage tanks, and other process equipment.
Volume and the Ideal Gas Law: A Deeper Look
The ideal gas law, PV = nRT, provides a powerful illustration of volume as an extensive property. The number of moles (n) directly relates to the amount of gas present. If you double the number of moles (keeping pressure and temperature constant), you'll double the volume. This showcases the direct proportionality between volume and the amount of matter (in this case, the amount of gas). The equation underscores the interconnectedness of volume with other properties like pressure and temperature, highlighting its significance in understanding gas behavior.
Real-World Applications of Volume Understanding
Beyond academic contexts, grasping volume's extensive nature has significant practical implications:
- Pharmaceutical industry: Accurate volume measurements are crucial in drug formulation, dosage, and dispensing.
- Food and beverage industry: Volume is essential for packaging, portion control, and process optimization.
- Environmental science: Volume estimations are used to understand water resources, pollution levels, and greenhouse gas emissions.
Conclusion: The Importance of Extensive Properties
Extensive properties like volume are not merely abstract concepts; they are fundamental to our understanding of the physical world and have far-reaching practical applications. Their ability to scale with the amount of matter provides a crucial link between macroscopic observations and microscopic behavior. Mastering the concepts of extensive properties, and specifically understanding how volume changes with the amount of substance, is vital for success in many scientific and engineering fields. The relationship between volume, mass, and density offers a foundational understanding of matter that expands into complex concepts across diverse disciplines, further emphasizing the importance of this seemingly simple, yet pervasive, property. Further exploration into these relationships will continue to unveil new insights and applications of extensive properties in various fields of study.
Latest Posts
Related Post
Thank you for visiting our website which covers about An Example Of Extensive Property Of Matter Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
