Are Freezing And Melting Point The Same
Muz Play
Mar 17, 2025 · 6 min read
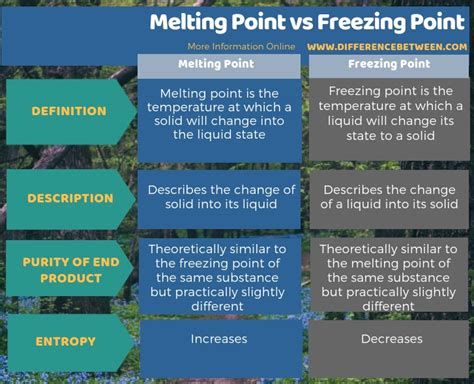
Table of Contents
Are Freezing and Melting Points the Same? Understanding Phase Transitions
The seemingly simple question, "Are freezing and melting points the same?" requires a more nuanced answer than a simple yes or no. While they represent the same temperature for a given substance under standard conditions, they describe fundamentally different processes in the context of phase transitions. This article delves deep into the concepts of freezing and melting, exploring the underlying physics, the subtle differences in their descriptions, and the factors that can influence these phase transitions.
Understanding Phase Transitions
Before diving into the specifics of freezing and melting, let's establish a foundational understanding of phase transitions. Phase transitions refer to the physical processes where a substance transforms from one state of matter to another, such as solid to liquid (melting), liquid to gas (boiling/vaporization), solid to gas (sublimation), and vice versa. These transitions are driven by changes in temperature and/or pressure, affecting the kinetic energy and intermolecular forces within the substance.
The Role of Temperature and Kinetic Energy
Temperature is a measure of the average kinetic energy of the particles (atoms, molecules, or ions) within a substance. At lower temperatures, the kinetic energy is low, and particles are relatively immobile, leading to a solid state characterized by a rigid structure. As temperature increases, the kinetic energy rises, allowing particles to overcome the intermolecular forces holding them in place, resulting in a transition to the liquid or gaseous phase.
The Influence of Intermolecular Forces
Intermolecular forces are the attractive forces between particles. These forces are responsible for holding the particles together in a solid state. The strength of these forces determines the melting and boiling points of a substance. Stronger intermolecular forces require higher temperatures to overcome them, resulting in higher melting and boiling points. For example, ice (water in its solid state) has a relatively low melting point compared to substances with stronger intermolecular forces, such as diamond.
Freezing: From Liquid to Solid
Freezing is the phase transition process where a liquid transforms into a solid. This occurs when the temperature of the liquid drops below its freezing point. As the temperature decreases, the kinetic energy of the particles diminishes, causing them to lose their mobility and become more ordered. This ordered arrangement is characteristic of the solid state, with particles tightly packed in a specific lattice structure.
The Freezing Point: A Critical Temperature
The freezing point is the specific temperature at which a liquid begins to solidify. It's crucial to understand that freezing is not an instantaneous process. As the liquid cools, it initially remains in a liquid state even below its freezing point, a phenomenon called supercooling. This happens because the liquid needs nucleation sites – imperfections or impurities – to initiate the formation of solid crystals. Once crystallization starts, the release of latent heat (the energy released during phase transitions) helps maintain the temperature at the freezing point until all the liquid is solidified.
Factors Affecting the Freezing Point
Several factors can influence the freezing point of a substance:
- Pressure: Increasing pressure generally increases the freezing point of a substance. However, this is not always the case, with water being a notable exception.
- Impurities: The presence of dissolved impurities in a liquid usually lowers its freezing point, a phenomenon known as freezing point depression. This is why adding salt to water lowers its freezing point, making it effective for de-icing roads and sidewalks.
- Crystallization: The formation of crystals is crucial to the freezing process. The rate of cooling, and thus crystal growth, significantly influence the resulting solid's properties. Slow cooling allows for the formation of large, well-defined crystals, while rapid cooling leads to the formation of smaller, less organized crystals.
Melting: From Solid to Liquid
Melting is the reverse process of freezing; it's the phase transition where a solid transforms into a liquid. This occurs when the temperature of the solid rises above its melting point. As the temperature increases, the kinetic energy of the particles increases, allowing them to overcome the intermolecular forces holding them in a fixed lattice structure. The particles gain sufficient energy to break free from their fixed positions and move more freely, resulting in a liquid state.
The Melting Point: The Temperature of Transition
The melting point is the specific temperature at which a solid begins to melt. Similar to freezing, melting is not instantaneous. The solid may remain solid slightly above its melting point – although superheating is less common than supercooling. This is due to the requirement of energy to break the bonds within the crystal lattice. The absorption of latent heat maintains the temperature at the melting point until all the solid is melted.
Factors Affecting the Melting Point
Similar to the freezing point, several factors can also affect the melting point of a substance:
- Pressure: Similar to freezing point, increasing pressure generally increases the melting point. Again, water is an exception.
- Impurities: The presence of impurities generally lowers the melting point. This is a useful technique in determining the purity of substances.
- Crystal Structure: The arrangement of particles in the solid's crystal lattice influences the melting point. Substances with more complex crystal structures generally have higher melting points.
Are Freezing and Melting Points Identical? The Equilibrium Point
For pure substances under standard conditions (1 atmosphere of pressure), the freezing and melting points are identical. This is because the transition between the solid and liquid phases occurs at the same temperature under equilibrium conditions. At the melting/freezing point, the rate of melting is equal to the rate of freezing. This represents a dynamic equilibrium where the solid and liquid phases coexist.
However, it's important to emphasize the distinction between the processes of freezing and melting. They are fundamentally opposite processes, even though they occur at the same temperature. Freezing involves the release of energy (exothermic), while melting requires the input of energy (endothermic). This difference in energy exchange is reflected in the sign of the enthalpy change (ΔH) associated with each process: ΔH is negative for freezing and positive for melting.
Exceptions and Considerations
While the freezing and melting points are usually identical for pure substances under standard pressure, certain factors can lead to discrepancies:
- Impurities: As mentioned, impurities lower both the freezing and melting points, but not necessarily by the same amount. The difference might be negligible in some cases but significant in others.
- Pressure: Changes in pressure can significantly impact the melting and freezing points, especially in substances with high compressibility. Water, for example, exhibits an unusual behavior where its freezing point decreases under increased pressure.
- Supercooling and Superheating: Supercooling and superheating can lead to temporary deviations from the expected freezing and melting points. These phenomena highlight the role of nucleation sites in phase transitions.
Conclusion: Understanding the Subtleties
While the freezing and melting points are numerically the same for a given pure substance under standard conditions, it's crucial to recognize that they represent distinct processes involving energy exchange and changes in molecular order. Freezing and melting describe the transitions between the solid and liquid phases from opposing perspectives—one involves the release of energy to establish a more ordered structure, the other the absorption of energy to disrupt this structure. Understanding the nuances of these phase transitions, including the influence of various factors like pressure and impurities, is essential in various fields, including materials science, chemistry, and meteorology. The seemingly simple question of whether freezing and melting points are the same unveils a complex interplay of temperature, energy, and intermolecular forces, highlighting the richness of physical phenomena.
Latest Posts
Latest Posts
-
Name A Structural Difference Between Triglycerides And Phospholipids
Mar 17, 2025
-
Ending Materials In A Chemical Reaction
Mar 17, 2025
-
Get Energy By Eating Other Organisms
Mar 17, 2025
-
What Is A Power Function In Math
Mar 17, 2025
-
How Many Phosphate Groups Does Atp Have
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Are Freezing And Melting Point The Same . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
