Are The Initial Velocities On An Uncompetitive Inhibitor The Same
Muz Play
Mar 26, 2025 · 5 min read
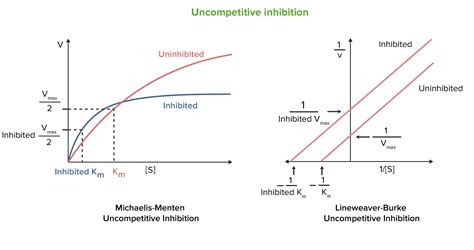
Table of Contents
Are the Initial Velocities on an Uncompetitive Inhibitor the Same? A Deep Dive into Enzyme Kinetics
Enzyme kinetics is a fundamental aspect of biochemistry, providing insights into enzyme mechanisms and cellular processes. Understanding how inhibitors affect enzyme activity is crucial for drug development and metabolic engineering. One type of inhibition, uncompetitive inhibition, presents a unique kinetic profile that often leads to confusion. A common question arising from studying uncompetitive inhibition is: are the initial velocities on an uncompetitive inhibitor the same? The answer, as we'll explore in detail, is no. But understanding why requires a closer look at the mechanism and the resulting kinetic equations.
Understanding Uncompetitive Inhibition
Unlike competitive inhibitors that bind to the free enzyme, uncompetitive inhibitors bind only to the enzyme-substrate complex (ES). This binding is often allosteric, meaning the inhibitor binds to a site different from the active site. This interaction alters the enzyme's conformation, preventing the release of the product and effectively halting the catalytic cycle. The key here is the dependency on the substrate being present for the inhibitor to exert its effect.
The Kinetic Mechanism of Uncompetitive Inhibition
The mechanism can be represented as follows:
- E + S ⇌ ES (Enzyme binds to substrate, forming the enzyme-substrate complex)
- ES + I ⇌ ESI (The enzyme-substrate complex binds to the inhibitor, forming an inactive ternary complex)
This differs significantly from competitive inhibition, where the inhibitor competes with the substrate for binding to the free enzyme. In uncompetitive inhibition, the inhibitor doesn't affect the enzyme directly; its effect is only apparent when the substrate is already bound.
The Impact on Initial Velocity
The initial velocity (V₀) of an enzymatic reaction is the rate of product formation at the very beginning of the reaction, before significant substrate depletion occurs. In the absence of an inhibitor, V₀ is governed by the Michaelis-Menten equation:
V₀ = Vmax[S] / (Km + [S])
Where:
- Vmax is the maximum velocity of the reaction
- Km is the Michaelis constant (an indicator of the enzyme's affinity for the substrate)
- [S] is the substrate concentration
However, the presence of an uncompetitive inhibitor alters this equation. The derivation, while complex, results in a modified Michaelis-Menten equation:
V₀ = Vmax)
Where:
- [I] is the inhibitor concentration
- Ki is the inhibitor dissociation constant (a measure of the inhibitor's affinity for the ES complex)
Notice the crucial difference: both Km and Vmax are effectively altered by the presence of the uncompetitive inhibitor. The apparent Km (Km') and Vmax' become:
- Km' = Km(1 + [I]/Ki)
- Vmax' = Vmax / (1 + [I]/Ki)
Why Initial Velocities are NOT the Same
From the modified Michaelis-Menten equation, it's clear that the initial velocity is directly impacted by the uncompetitive inhibitor's concentration. The presence of the inhibitor lowers both the apparent Vmax and the apparent Km proportionally. This means that at any given substrate concentration, the initial velocity in the presence of an uncompetitive inhibitor will be lower than the initial velocity in its absence.
This is a key distinction from competitive inhibition, where the Vmax remains unchanged. The different effects on Vmax highlight the fundamentally different mechanisms of action.
Graphical Representation and Analysis
The Lineweaver-Burk plot (a double reciprocal plot of 1/V₀ vs 1/[S]) provides a visual way to distinguish between different types of inhibition.
- Competitive Inhibition: Lines intersect on the y-axis (same Vmax, different Km).
- Uncompetitive Inhibition: Lines are parallel (different Vmax and Km, with a proportional change).
- Mixed Inhibition: Lines intersect to the left of the y-axis (different Vmax and Km, with a non-proportional change).
This parallel nature of the Lineweaver-Burk plot for uncompetitive inhibition reinforces the observation that both Vmax and Km are affected proportionately. This visual representation clearly demonstrates that the initial velocities are not the same in the presence and absence of an uncompetitive inhibitor.
Practical Implications and Examples
Understanding uncompetitive inhibition is not just a theoretical exercise; it has significant practical implications in various fields:
-
Drug Design: Many drugs act as uncompetitive inhibitors, targeting specific enzyme-substrate complexes to disrupt metabolic pathways. By understanding the kinetics, researchers can design more effective drugs with higher affinity and specificity.
-
Metabolic Engineering: Manipulating enzyme activity through uncompetitive inhibitors can be used to optimize metabolic pathways in industrial biotechnology. This approach allows for fine-tuning of metabolic fluxes and improving the production of desired compounds.
-
Diagnostic Applications: The distinct kinetic profile of uncompetitive inhibition can be exploited in diagnostic assays to identify and quantify specific enzymes or their inhibitors.
Examples of Uncompetitive Inhibition in Biological Systems:
While pinpointing specific examples with concrete Ki and Km values that are readily available in the literature can be challenging without delving into complex research papers, many enzyme systems exhibit uncompetitive inhibition patterns. For instance, certain lipolytic enzymes show uncompetitive inhibition in the presence of specific inhibitors. Many drug targets also exhibit this type of inhibition, highlighting its biological relevance.
Addressing Common Misconceptions
The apparent simplicity of the uncompetitive inhibition mechanism can sometimes lead to misunderstandings:
-
The inhibitor doesn't directly compete with the substrate: This is crucial. The inhibitor only binds to the ES complex; it cannot bind to the free enzyme.
-
Both Vmax and Km are affected: This is unlike competitive inhibition, where only Km is affected. The proportional change in both parameters is the hallmark of uncompetitive inhibition.
-
Initial velocities are always lower: At any given substrate concentration, the initial velocity will always be lower in the presence of an uncompetitive inhibitor compared to its absence.
Conclusion
In summary, the initial velocities in an enzymatic reaction are not the same in the presence and absence of an uncompetitive inhibitor. The uncompetitive inhibitor alters both the apparent Vmax and Km proportionally, leading to a lower initial velocity at any given substrate concentration. Understanding this distinction is critical for interpreting enzyme kinetics data and designing effective strategies in drug development, metabolic engineering, and diagnostic applications. The unique characteristics of uncompetitive inhibition set it apart from other types of inhibition, providing valuable insights into enzyme mechanisms and their regulation within biological systems. The parallel lines observed in the Lineweaver-Burk plot serve as a visual confirmation of this phenomenon, further solidifying the understanding that uncompetitive inhibitors profoundly affect the initial velocities of enzyme-catalyzed reactions. The effects are clearly demonstrated by the modified Michaelis-Menten equation, highlighting the mathematical basis behind this distinct form of enzyme inhibition.
Latest Posts
Latest Posts
-
How To Calculate Confidence Interval Without Standard Deviation
Mar 29, 2025
-
How Much Force To Break A Femur
Mar 29, 2025
-
Find The Equation Of A Line Shown
Mar 29, 2025
-
Why Does Active Transport Require Energy
Mar 29, 2025
-
Cellulose And Starch Are Examples Of
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Are The Initial Velocities On An Uncompetitive Inhibitor The Same . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
