Atom That Has Gained Or Lost Electrons
Muz Play
Mar 31, 2025 · 6 min read
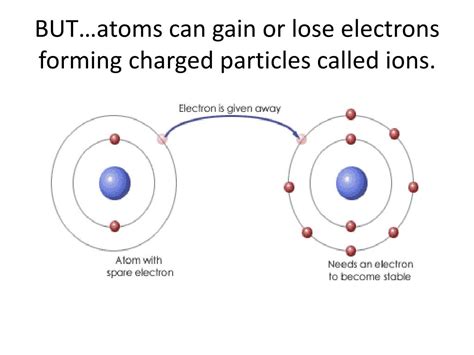
Table of Contents
Ions: Atoms That Have Gained or Lost Electrons
Atoms, the fundamental building blocks of matter, are incredibly fascinating entities. Their behavior, particularly their tendency to gain or lose electrons, forms the basis for countless chemical reactions and determines the properties of the materials we interact with daily. When an atom deviates from its neutral state by acquiring or shedding electrons, it transforms into an ion, a charged particle that plays a crucial role in diverse phenomena, from the formation of ionic compounds to the functioning of biological systems. This article delves into the world of ions, exploring their formation, properties, and significance in various contexts.
Understanding Atomic Structure and Electron Behavior
Before we delve into the specifics of ion formation, let's refresh our understanding of basic atomic structure. An atom comprises a central nucleus containing positively charged protons and neutral neutrons. Surrounding the nucleus are negatively charged electrons, which reside in specific energy levels or shells. The number of protons defines the atomic number of an element and determines its identity. In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
Electrons, however, are not rigidly fixed in their orbits. They can be excited to higher energy levels or even completely removed from the atom, depending on the energy involved. This ability of electrons to be transferred, especially the valence electrons in the outermost shell, is the key to understanding ion formation.
The Formation of Ions: Gaining and Losing Electrons
Ions are formed when an atom either gains or loses electrons, resulting in an imbalance between the number of protons and electrons. This imbalance creates a net electrical charge.
Cations: Positively Charged Ions
When an atom loses one or more electrons, it becomes positively charged. These positively charged ions are known as cations. The loss of electrons usually occurs because the atom is more stable with fewer electrons, especially when it involves the outermost electron shell. The number of electrons lost determines the magnitude of the positive charge. For instance, a sodium atom (Na) readily loses one electron to form a sodium ion (Na⁺), while a calcium atom (Ca) loses two electrons to form a calcium ion (Ca²⁺).
The driving force behind cation formation is the atom's desire to achieve a stable electron configuration. Many atoms strive to achieve a full outermost electron shell, a state often referred to as the octet rule (eight electrons in the outermost shell). By losing electrons, these atoms achieve a more stable, lower-energy configuration.
Anions: Negatively Charged Ions
Conversely, when an atom gains one or more electrons, it becomes negatively charged, forming an anion. This gain of electrons often results in a more stable electron configuration, usually by completing the outermost electron shell. For example, a chlorine atom (Cl) readily gains one electron to form a chloride ion (Cl⁻), while an oxygen atom (O) gains two electrons to form an oxide ion (O²⁻).
The process of anion formation is driven by the atom's electronegativity, which is its tendency to attract electrons. Highly electronegative atoms readily gain electrons to achieve a stable electron configuration.
Properties of Ions
The formation of ions drastically alters the properties of atoms. These changes are primarily due to the change in the net charge and the consequent effect on the atom's electron configuration:
-
Charge: The most significant difference is the presence of a net electrical charge. Cations are positively charged, while anions are negatively charged. This charge significantly influences the ion's interactions with other charged particles.
-
Size: Ionic size differs from the neutral atom's size. Cations are generally smaller than their corresponding neutral atoms because the loss of electrons reduces electron-electron repulsion and allows the remaining electrons to be drawn closer to the nucleus. Anions, on the other hand, are generally larger than their neutral atoms because the addition of electrons increases electron-electron repulsion, causing the electrons to occupy a larger space.
-
Reactivity: Ions are highly reactive, especially compared to their neutral counterparts. Their charge allows them to participate in a wide range of chemical reactions, often forming ionic compounds.
-
Physical Properties: Ionic compounds, formed by the electrostatic attraction between cations and anions, possess unique physical properties distinct from covalent compounds. They tend to have high melting and boiling points, are often brittle, and are frequently soluble in water.
The Significance of Ions in Various Contexts
Ions play pivotal roles in various scientific disciplines and everyday phenomena:
Chemistry: Ionic Bonding and Compound Formation
Ionic bonding is a type of chemical bond formed by the electrostatic attraction between oppositely charged ions (cations and anions). This strong electrostatic attraction results in the formation of ionic compounds, such as sodium chloride (NaCl), commonly known as table salt. The properties of ionic compounds are significantly determined by the nature of the ions involved.
Biology: Essential Roles in Biological Processes
Ions are crucial for many biological processes. For instance:
-
Electrolyte Balance: Ions such as sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) are vital electrolytes in our bodies. They maintain fluid balance, transmit nerve impulses, and contract muscles. Imbalances in electrolyte levels can have serious health consequences.
-
Enzyme Function: Many enzymes require specific ions as cofactors to function properly. These ions play critical roles in catalyzing biochemical reactions.
-
Cellular Processes: Ion gradients across cell membranes are essential for processes such as active transport, nerve impulse transmission, and muscle contraction.
Physics: Applications in various fields
Ions are also important in various physics applications, including:
-
Mass Spectrometry: Mass spectrometry uses ions to determine the mass-to-charge ratio of molecules, a technique widely used in analytical chemistry and proteomics.
-
Plasma Physics: Plasmas, which are ionized gases, are studied in various areas of physics, including astrophysics and fusion research.
-
Ion Propulsion: Ion propulsion systems use accelerated ions to generate thrust, enabling efficient spacecraft propulsion.
Identifying and Analyzing Ions
Several techniques can be used to identify and analyze ions:
-
Spectroscopy: Various spectroscopic techniques, such as atomic emission spectroscopy and mass spectrometry, can identify ions based on their unique spectral signatures.
-
Electrochemical Methods: Electrochemical techniques like potentiometry and voltammetry can measure ion concentrations and determine their properties.
-
Chromatographic Techniques: Chromatographic methods can separate and identify different ions in a mixture.
Conclusion
Ions, formed by the gain or loss of electrons, are ubiquitous in nature and play crucial roles in various aspects of our world. Understanding their formation, properties, and significance is essential across diverse scientific disciplines, from chemistry and biology to physics and materials science. Their importance in chemical reactions, biological processes, and various technological applications underscores their fundamental role in shaping the world around us. The continued study of ions promises further insights into the intricacies of matter and its interactions, leading to new discoveries and technological advancements.
Latest Posts
Latest Posts
-
Root Mean Square Velocity Of Gas
Apr 01, 2025
-
Where Is The Respiratory Center Located In The Brain
Apr 01, 2025
-
What Is The Difference Between Cellular Respiration And Fermentation
Apr 01, 2025
-
What Are Two Functional Groups Found In Amino Acids
Apr 01, 2025
-
Foundations Of Education 13th Edition Pdf Free Download
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Atom That Has Gained Or Lost Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
