Atomic Mass Unit Vs Molar Mass
Muz Play
Mar 25, 2025 · 5 min read
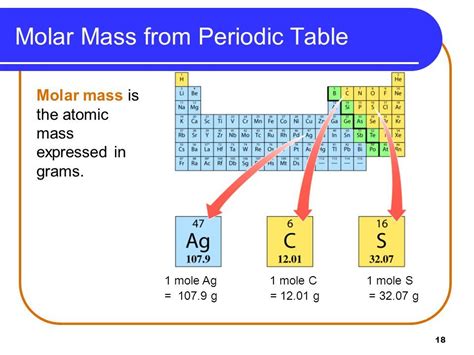
Table of Contents
Atomic Mass Unit vs. Molar Mass: Understanding the Difference
The concepts of atomic mass unit (amu) and molar mass are fundamental in chemistry, crucial for understanding the composition and properties of matter. While closely related, they represent different aspects of mass at different scales. This article delves into the distinctions between atomic mass units and molar mass, clarifying their meanings, applications, and the relationship between them.
What is an Atomic Mass Unit (amu)?
An atomic mass unit (amu), also known as a dalton (Da), is a unit of mass used to express the mass of atoms and molecules. It's defined as one-twelfth the mass of a single carbon-12 atom (¹²C). This means that a carbon-12 atom has a mass of exactly 12 amu. All other atomic masses are relative to this standard.
Significance of the Carbon-12 Standard
The choice of carbon-12 as the standard is not arbitrary. Carbon-12 is relatively abundant and readily available, making it a convenient reference point. Its nuclear structure is also well-understood and stable. Using this standard allows for consistent and accurate measurements of atomic masses across different laboratories and experiments.
Calculating Atomic Mass
The atomic mass of an element is the weighted average of the masses of its isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. For instance, chlorine exists as two main isotopes: ³⁵Cl and ³⁷Cl. The atomic mass of chlorine is a weighted average reflecting the abundance of each isotope. This weighted average is expressed in amu.
For example: If 75% of chlorine atoms are ³⁵Cl (mass ≈ 35 amu) and 25% are ³⁷Cl (mass ≈ 37 amu), the average atomic mass would be:
(0.75 * 35 amu) + (0.25 * 37 amu) ≈ 35.5 amu
Applications of Atomic Mass Unit
The amu is primarily used in:
- Determining the mass of individual atoms and molecules: This is crucial for understanding the stoichiometry of chemical reactions and the structure of molecules.
- Mass spectrometry: This analytical technique measures the mass-to-charge ratio of ions, enabling the identification and quantification of different molecules. The results are often expressed in amu.
- Nuclear physics: The amu is vital in calculations involving nuclear reactions and radioactive decay.
What is Molar Mass?
Molar mass is the mass of one mole of a substance. A mole (mol) is a unit of measurement representing a specific number of particles, defined as Avogadro's number (approximately 6.022 x 10²³). This means one mole of any substance contains Avogadro's number of particles (atoms, molecules, ions, etc.).
The Significance of Avogadro's Number
Avogadro's number provides a link between the microscopic world of atoms and molecules and the macroscopic world of measurable quantities. It allows chemists to work with large numbers of atoms and molecules in a practical and manageable way.
Calculating Molar Mass
The molar mass of an element is numerically equal to its atomic mass, but the units are different. The atomic mass is expressed in amu, while the molar mass is expressed in grams per mole (g/mol).
For example:
- The atomic mass of carbon is approximately 12 amu.
- The molar mass of carbon is approximately 12 g/mol.
For compounds, the molar mass is the sum of the molar masses of all the atoms in the chemical formula.
For example: The molar mass of water (H₂O) is calculated as follows:
- Molar mass of H = 1 g/mol
- Molar mass of O = 16 g/mol
- Molar mass of H₂O = (2 * 1 g/mol) + (1 * 16 g/mol) = 18 g/mol
Applications of Molar Mass
Molar mass is essential for various chemical calculations, including:
- Stoichiometry: Determining the amounts of reactants and products in chemical reactions.
- Concentration calculations: Expressing the concentration of solutions in units such as molarity (moles per liter).
- Titrations: Determining the concentration of an unknown solution by reacting it with a solution of known concentration.
- Gas law calculations: Relating the volume, pressure, and temperature of gases to the number of moles.
The Relationship Between Atomic Mass Unit and Molar Mass
The key relationship lies in Avogadro's number. The molar mass of a substance is simply the atomic mass (or molecular mass) expressed in grams per mole instead of amu. This means that one mole of a substance contains Avogadro's number of particles, and the mass of one mole is numerically equivalent to the atomic (or molecular) mass in grams.
This conversion allows for seamless transition between the microscopic and macroscopic scales in chemical calculations.
Common Mistakes and Misconceptions
Several common misconceptions surround atomic mass units and molar mass:
- Confusing amu and g/mol: While numerically equal for single elements, the units represent different concepts. Amu represents the mass of a single atom or molecule, whereas g/mol represents the mass of Avogadro's number of atoms or molecules.
- Ignoring isotopes: Failing to consider the isotopic composition of an element can lead to inaccurate calculations of atomic and molar mass.
- Incorrectly calculating molar mass of compounds: Errors often occur in adding the molar masses of the constituent elements in a compound.
Advanced Concepts and Applications
The concepts of amu and molar mass extend beyond basic stoichiometry. They are fundamental to more advanced topics like:
- Thermodynamics: Molar mass is used in calculating enthalpy, entropy, and Gibbs free energy changes for chemical reactions.
- Kinetics: Molar mass influences reaction rates and diffusion processes.
- Spectroscopy: Interpreting spectroscopic data often requires understanding the relationship between mass and molecular structure.
Conclusion
Atomic mass units (amu) and molar mass are intertwined yet distinct concepts crucial to understanding the quantitative aspects of chemistry. While amu focuses on the mass of individual atoms and molecules, molar mass bridges the microscopic and macroscopic scales, allowing chemists to perform practical calculations involving large numbers of particles. Understanding the relationship between these units, along with their respective applications, is fundamental to mastering many chemical concepts and solving problems in various fields. By carefully considering the units and their significance, you can avoid common mistakes and unlock a deeper understanding of the composition and behavior of matter.
Latest Posts
Latest Posts
-
The Blank Atom In R 12 Is Believed To Brea Off
Mar 28, 2025
-
Heat Of Neutralization For Hcl And Naoh
Mar 28, 2025
-
Difference Between Selective Media And Differential Media
Mar 28, 2025
-
How To Calculate Exerting Force With Center Of Mass
Mar 28, 2025
-
Which Of The Following Molecules Are Chiral
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Atomic Mass Unit Vs Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
