Atoms That Have Gained Or Lost Electrons
Muz Play
Mar 29, 2025 · 7 min read
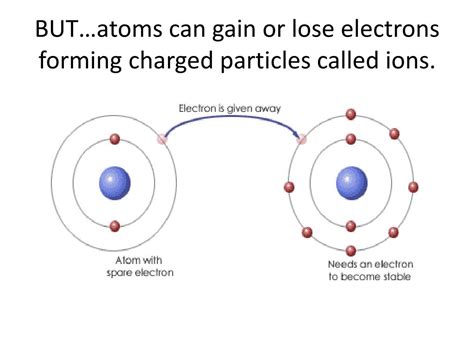
Table of Contents
Atoms That Have Gained or Lost Electrons: Ions and Their Significance
Atoms, the fundamental building blocks of matter, are typically depicted as neutral entities, possessing an equal number of positively charged protons in their nucleus and negatively charged electrons orbiting around it. However, this neutral state isn't always the case. Atoms can readily gain or lose electrons, transforming into charged particles known as ions. This seemingly simple process has profound implications across various scientific disciplines, impacting everything from the formation of chemical bonds to the functioning of biological systems. Understanding ions is crucial to grasping the intricacies of chemistry, physics, and even biology.
The Formation of Ions: Gaining and Losing Electrons
The driving force behind ion formation is the quest for stability. Atoms strive to achieve a full outer electron shell, a configuration that mirrors the exceptionally stable electron arrangement of noble gases. This principle, known as the octet rule, dictates that atoms tend to gain, lose, or share electrons to attain eight electrons in their outermost shell. Exceptions exist, particularly with elements in the first and second periods of the periodic table, which may strive for a duet (two electrons) or octet.
Cation Formation: The Loss of Electrons
When an atom loses one or more electrons, it becomes positively charged, forming a cation. This is because the number of protons (positive charges) now exceeds the number of electrons (negative charges). Metals, located on the left side of the periodic table, are particularly prone to losing electrons. They typically have relatively few electrons in their outermost shell, making it energetically favorable to relinquish these electrons and achieve a more stable configuration.
For instance, a sodium atom (Na) has one electron in its outermost shell. Losing this single electron transforms it into a sodium cation (Na⁺), achieving a stable electron configuration matching that of neon (Ne). Similarly, magnesium (Mg), with two electrons in its outermost shell, readily loses both to form the Mg²⁺ cation. The number of electrons lost dictates the magnitude of the positive charge on the cation.
The ease with which an atom loses electrons is quantified by its ionization energy. This is the energy required to remove an electron from a gaseous atom. Lower ionization energies indicate a greater tendency to lose electrons and form cations.
Anion Formation: The Gain of Electrons
Conversely, when an atom gains one or more electrons, it becomes negatively charged, forming an anion. In this case, the number of electrons (negative charges) now surpasses the number of protons (positive charges). Nonmetals, situated on the right side of the periodic table, have a greater affinity for electrons and readily gain electrons to achieve a stable electron configuration.
Consider a chlorine atom (Cl), which has seven electrons in its outermost shell. Gaining one electron fills this shell, transforming chlorine into a chloride anion (Cl⁻), mirroring the stable electron configuration of argon (Ar). Similarly, oxygen (O), with six electrons in its outermost shell, gains two electrons to form the oxide anion (O²⁻).
The electron affinity measures an atom's ability to attract an electron. A higher electron affinity indicates a greater tendency to gain electrons and form anions.
The Role of Ions in Chemical Bonding
The formation of ions is paramount to the creation of ionic bonds, a fundamental type of chemical bond. Ionic bonds arise from the electrostatic attraction between oppositely charged ions. The strong attraction between a cation and an anion forms a stable ionic compound.
For example, the reaction between sodium (Na) and chlorine (Cl) leads to the formation of sodium chloride (NaCl), common table salt. Sodium loses an electron to become Na⁺, and chlorine gains an electron to become Cl⁻. The electrostatic attraction between these oppositely charged ions forms the ionic bond that holds the sodium chloride crystal lattice together.
This process is not limited to simple binary compounds. Many complex ionic compounds exist, involving multiple cations and anions interacting through electrostatic forces.
Ions in Biological Systems: Crucial Roles in Life
Ions play a critical role in numerous biological processes. Their charges and interactions are essential for maintaining proper cellular function and overall organismal health.
Electrolyte Balance: Maintaining Cellular Function
Electrolytes, which are ions dissolved in bodily fluids, are vital for maintaining the proper osmotic balance within cells. They influence water movement across cell membranes and contribute to the regulation of cell volume. Key electrolytes include sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻). Imbalances in electrolyte concentrations can lead to severe health problems.
Nerve Impulse Transmission: The Role of Ions in Signaling
Ions are crucial for nerve impulse transmission. The movement of sodium (Na⁺) and potassium (K⁺) ions across the neuronal membrane generates electrical signals that propagate nerve impulses throughout the nervous system. This precise control of ion flow allows for rapid communication within the body.
Muscle Contraction: Ions Drive Movement
Similarly, ions are essential for muscle contraction. Calcium (Ca²⁺) ions play a critical role in initiating the cascade of events that lead to muscle fiber shortening and movement. The precise regulation of calcium ion concentration is crucial for coordinated muscle contractions.
Enzyme Function: Ions as Cofactors
Many enzymes, biological catalysts, require ions as cofactors to function properly. These ions may participate directly in the catalytic process or stabilize the enzyme's three-dimensional structure. Magnesium (Mg²⁺), zinc (Zn²⁺), and iron (Fe²⁺/Fe³⁺) are examples of ions that commonly serve as enzymatic cofactors.
Ions and Their Applications in Various Fields
The properties of ions have led to their widespread application in diverse fields.
Industrial Applications: Electroplating and Corrosion Protection
Ions are used extensively in electroplating, a process where a thin layer of metal is deposited onto a surface using an electric current. This technique enhances the corrosion resistance and aesthetic appeal of various materials.
Medical Applications: Diagnostic Tools and Treatments
Ions play a critical role in medical diagnostics and treatments. Blood electrolyte analysis provides insights into the overall health status of patients. Radioactive isotopes, which are ions with unstable nuclei, are utilized in medical imaging techniques such as PET scans and radiation therapy for cancer treatment.
Environmental Applications: Water Treatment and Soil Remediation
Ion exchange processes are crucial for water treatment and soil remediation. These processes utilize ion exchange resins to remove undesirable ions from water or soil, improving water quality and addressing environmental pollution.
Beyond the Octet Rule: Exceptions and Complexities
While the octet rule provides a useful framework for understanding ion formation, it's important to acknowledge exceptions. Transition metals, located in the d-block of the periodic table, often exhibit variable oxidation states, meaning they can form ions with varying charges. This is because they can lose electrons from both their outermost s and d orbitals. Furthermore, some elements can form ions with electron configurations that deviate from the octet rule.
Detecting and Analyzing Ions: Techniques and Methods
Several techniques are employed to detect and analyze ions. These include:
- Flame tests: Heating a sample in a flame produces characteristic colors that identify specific metal ions.
- Spectroscopy: Analyzing the absorption or emission of light by ions can reveal their identity and concentration.
- Chromatography: Separating ions based on their properties allows for individual identification and quantification.
- Electrochemical methods: Measuring electrical conductivity or potential differences can provide information about ion concentration and activity.
Conclusion: The Ubiquity and Importance of Ions
The formation of ions, through the gain or loss of electrons, is a fundamental process that profoundly impacts the chemical and physical properties of matter. From the formation of simple ionic compounds to the complex biochemical reactions within living organisms, ions play a crucial and ubiquitous role. Understanding their behavior and properties is essential for advancements in chemistry, physics, biology, medicine, and many other scientific and technological fields. The continuing exploration of ionic interactions will undoubtedly reveal further insights into the intricacies of the natural world and open new avenues for technological innovation.
Latest Posts
Latest Posts
-
Are All Ionic Compounds Strong Electrolytes
Apr 01, 2025
-
How Do You Use A Balance
Apr 01, 2025
-
Nucleotide Excision Repair Vs Mismatch Repair
Apr 01, 2025
-
What Is The Difference Between Religious And Ethnic Groups
Apr 01, 2025
-
What Is Found In Both Dna And Rna
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Atoms That Have Gained Or Lost Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
