Can P Orbitals Form Sigma Bonds
Muz Play
Apr 07, 2025 · 6 min read
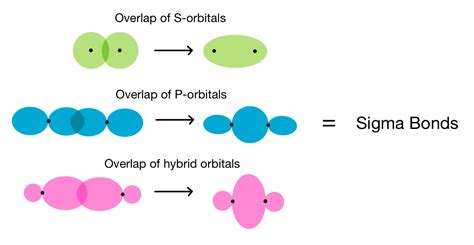
Table of Contents
Can p Orbitals Form Sigma Bonds? A Deep Dive into Molecular Orbital Theory
The question of whether p orbitals can form sigma bonds is a fundamental concept in chemistry, often sparking confusion among students. While the image of s orbitals forming sigma bonds is readily accepted, the role of p orbitals in sigma bonding requires a more nuanced understanding of orbital hybridization and molecular orbital theory. This article will delve deep into this topic, exploring the conditions under which p orbitals participate in sigma bond formation and highlighting the complexities involved.
Understanding Sigma and Pi Bonds
Before we address the core question, let's establish a clear understanding of sigma (σ) and pi (π) bonds. These terms describe the different types of covalent bonds formed through the overlap of atomic orbitals.
Sigma (σ) Bonds: Head-on Overlap
Sigma bonds are characterized by head-on overlap of atomic orbitals. This means the electron density is concentrated along the internuclear axis—the imaginary line connecting the two bonded atoms. This type of overlap results in a strong, stable bond. S orbitals always form sigma bonds because their spherical shape allows for direct head-on overlap with other orbitals.
Pi (π) Bonds: Side-on Overlap
Pi bonds, on the other hand, involve side-on overlap of atomic orbitals. The electron density is concentrated above and below the internuclear axis, resulting in a weaker bond compared to sigma bonds. Pi bonds are typically formed by the overlap of p orbitals (or d orbitals in some cases).
How p Orbitals Contribute to Sigma Bond Formation
The straightforward answer is: yes, p orbitals can form sigma bonds, but not always directly in their unhybridized state. The formation of a sigma bond using p orbitals requires specific circumstances and often involves hybridization.
1. Direct Overlap (Less Common):
In simpler diatomic molecules like oxygen (O₂), the sigma bond can be formed from the direct head-on overlap of two p orbitals. However, this scenario is relatively less common compared to those involving hybridized orbitals. The pure p orbital overlap in O₂ is a good example, leading to the double bond (one sigma and one pi).
2. Hybridization: The Key Role
Hybridization is a crucial process that significantly influences the ability of p orbitals to form sigma bonds. It's a mathematical model explaining how atomic orbitals combine to form hybrid orbitals with different shapes and energies, optimizing the bonding possibilities. The most common types of hybridization involving p orbitals are:
-
sp Hybridization: One s orbital and one p orbital combine to form two sp hybrid orbitals oriented linearly (180° apart). Each sp hybrid orbital can form a sigma bond. Examples include acetylene (C₂H₂), where the carbon atoms form a triple bond (one sigma and two pi bonds), with the sigma bond arising from the sp hybrid orbital overlap.
-
sp² Hybridization: One s orbital and two p orbitals combine to form three sp² hybrid orbitals arranged in a trigonal planar geometry (120° apart). Each sp² hybrid orbital can form a sigma bond. Examples include ethylene (C₂H₄), where the carbon atoms have a double bond (one sigma and one pi bond), with the sigma bond arising from the overlap of sp² hybrid orbitals.
-
sp³ Hybridization: One s orbital and three p orbitals combine to form four sp³ hybrid orbitals arranged in a tetrahedral geometry (109.5° apart). Each sp³ hybrid orbital can form a sigma bond. Methane (CH₄) is a classic example, with each C-H bond being a sigma bond formed by the overlap of an sp³ hybrid orbital from carbon and an s orbital from hydrogen.
Therefore, in most cases, p orbitals contribute to sigma bond formation indirectly through hybridization. The hybridized orbitals, with their optimized shapes and orientations, are better suited for effective head-on overlap and stronger sigma bond formation.
Understanding the Exceptions and Complexities
While hybridization offers a powerful framework to understand sigma bond formation involving p orbitals, there are exceptions and complexities to consider:
-
Limitations of Hybridization: Hybridization is a model, a convenient simplification of the complex interactions within atoms. It doesn't fully represent the actual electron distribution. In some cases, especially in larger or more complex molecules, relying solely on hybridization may provide an incomplete picture.
-
Molecules without Hybridization: Some molecules exhibit bonding patterns that cannot be easily explained by traditional hybridization schemes. Advanced theoretical methods beyond the scope of simple hybridization models are often required to understand the bonding in these cases.
-
Influence of Molecular Geometry: Molecular geometry strongly influences the type of bonds formed. Linear, trigonal planar, and tetrahedral geometries are strongly associated with sp, sp², and sp³ hybridization, respectively. Deviations from these ideal geometries can affect the type of overlap and therefore the strength of sigma bonds.
-
The Role of Molecular Orbital Theory: Molecular orbital theory (MOT) offers a more complete and accurate description of bonding than simple hybridization. MOT considers the interaction of all atomic orbitals in a molecule, generating molecular orbitals that extend over the entire molecule. This approach allows for a more precise depiction of bond order, bond strength, and electron distribution in molecules where sigma bonds involve p orbitals. It accurately explains the subtle differences in bond properties that hybridization alone might miss.
Illustrative Examples: Different Hybridizations and Sigma Bonds
Let's analyze several examples to illustrate how p orbitals contribute to sigma bond formation in different hybridization scenarios:
1. Ethene (C₂H₄):
- Carbon Hybridization: sp²
- Sigma Bonds: The C=C double bond contains one sigma bond formed by the head-on overlap of two sp² hybrid orbitals from each carbon atom. Each carbon also forms two sigma bonds with hydrogen atoms, utilizing the remaining two sp² hybrid orbitals on each carbon atom.
- Pi Bonds: The second bond in the C=C double bond is a pi bond, formed by the side-on overlap of two unhybridized p orbitals.
2. Ethane (C₂H₆):
- Carbon Hybridization: sp³
- Sigma Bonds: Each carbon atom forms four sigma bonds—one with the other carbon atom (formed by the overlap of sp³ orbitals) and three with hydrogen atoms (formed by the overlap of sp³ orbitals on carbon and s orbitals on hydrogen).
3. Acetylene (C₂H₂):
- Carbon Hybridization: sp
- Sigma Bonds: The C≡C triple bond contains one sigma bond formed by the head-on overlap of two sp hybrid orbitals from each carbon atom. Each carbon also forms one sigma bond with a hydrogen atom, utilizing the remaining sp hybrid orbital.
- Pi Bonds: The other two bonds in the triple bond are pi bonds, formed by the side-on overlap of two sets of unhybridized p orbitals.
Conclusion: A Multifaceted Perspective
The question of whether p orbitals can form sigma bonds is best answered with a nuanced understanding of orbital hybridization and molecular orbital theory. While direct head-on overlap of unhybridized p orbitals can contribute to sigma bonding in some cases (like O₂), hybridization significantly enhances and optimizes the sigma bond formation process. Hybridized orbitals, with their tailored shapes and orientations, facilitate more effective head-on overlaps, leading to stronger and more stable sigma bonds. While hybridization serves as a valuable model, molecular orbital theory offers a more complete and accurate description of bonding in molecules where sigma bonds involve p orbitals, especially when dealing with complexities not easily captured by hybridization alone. Ultimately, a comprehensive understanding involves appreciating both approaches and their respective strengths and limitations.
Latest Posts
Latest Posts
-
Three Steps Of The Perception Process
Apr 09, 2025
-
How To Tell If Something Is Axial Or Equatorial
Apr 09, 2025
-
Is Pool Water A Homogeneous Mixture
Apr 09, 2025
-
Fungi Can Reproduce Both Sexually And Asexually
Apr 09, 2025
-
What Is The Driving Force Of Evolution
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Can P Orbitals Form Sigma Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.