Chemical Families In The Periodic Table
Muz Play
Mar 29, 2025 · 6 min read
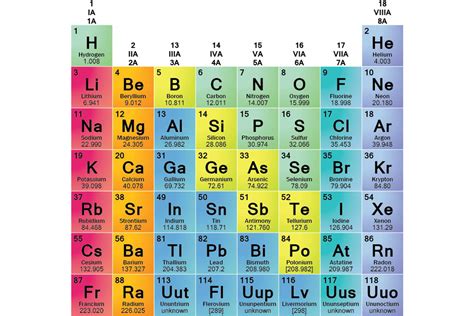
Table of Contents
Chemical Families in the Periodic Table: A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. Understanding these properties allows us to group elements into chemical families, also known as groups. These families exhibit similar chemical behaviors due to shared valence electron configurations, leading to predictable reactivity patterns. This comprehensive exploration delves into the major chemical families, their characteristics, and their significance in various applications.
The Alkali Metals (Group 1)
The alkali metals, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), are highly reactive metals found in Group 1. Their defining characteristic is possessing one valence electron. This single electron is easily lost, resulting in a +1 oxidation state and highly reactive behavior.
Key Properties of Alkali Metals:
- Low density: They are the least dense metals.
- Low melting and boiling points: Compared to other metals, they have significantly lower melting and boiling points.
- Softness: They are soft enough to be cut with a knife.
- High reactivity: They readily react with water, oxygen, and halogens, often explosively.
- Formation of ionic compounds: They readily form ionic compounds with non-metals.
Applications of Alkali Metals:
- Lithium: Used in rechargeable batteries for electronic devices and electric vehicles due to its high energy density. It also finds applications in ceramics and glass manufacturing.
- Sodium: Essential for human life, sodium compounds are used extensively in the food industry (sodium chloride – common salt), and in the production of various chemicals. Sodium vapor lamps are used for street lighting.
- Potassium: Crucial for plant growth and plays a vital role in human physiology. Potassium compounds are used in fertilizers and various industrial processes.
The Alkaline Earth Metals (Group 2)
Group 2 comprises the alkaline earth metals: beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). These metals possess two valence electrons, leading to a +2 oxidation state and relatively high reactivity, although less than the alkali metals.
Key Properties of Alkaline Earth Metals:
- Higher density than alkali metals: They are denser than alkali metals.
- Higher melting and boiling points than alkali metals: They have higher melting and boiling points compared to alkali metals.
- Moderately reactive: They react with oxygen and water, although less vigorously than alkali metals.
- Formation of ionic compounds: They readily form ionic compounds with non-metals.
Applications of Alkaline Earth Metals:
- Magnesium: Used in lightweight alloys for aircraft and automobiles due to its high strength-to-weight ratio. It's also used in flash photography and pyrotechnics.
- Calcium: Essential for bone formation in animals and is used in various building materials (cement, plaster). Calcium compounds are used in fertilizers and various industrial applications.
- Strontium: Used in fireworks to produce a brilliant red color.
The Transition Metals (Groups 3-12)
The transition metals occupy the central block of the periodic table (Groups 3-12). Their defining characteristic is the partially filled d orbitals in their valence shells. This results in a variety of oxidation states and complex chemical behavior.
Key Properties of Transition Metals:
- High melting and boiling points: They generally have high melting and boiling points.
- High density: They are typically dense metals.
- Variable oxidation states: They can exhibit multiple oxidation states.
- Formation of colored compounds: Many of their compounds are brightly colored.
- Catalytic activity: Many transition metals and their compounds act as catalysts in various chemical reactions.
Applications of Transition Metals:
- Iron (Fe): Widely used in steel and other alloys due to its strength and low cost. It plays a crucial role in biological systems (hemoglobin).
- Copper (Cu): Excellent conductor of electricity, used in electrical wiring and plumbing. It's also used in alloys such as brass and bronze.
- Gold (Au) and Silver (Ag): Highly valued precious metals used in jewelry, coinage, and electronics due to their inertness and conductivity.
- Platinum (Pt) and Palladium (Pd): Used as catalysts in various industrial processes, including automobile catalytic converters.
The Halogens (Group 17)
The halogens – fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) – are highly reactive non-metals found in Group 17. They possess seven valence electrons and readily gain one electron to achieve a stable octet, resulting in a -1 oxidation state.
Key Properties of Halogens:
- High electronegativity: They are highly electronegative, meaning they strongly attract electrons.
- Highly reactive: They readily react with metals to form ionic compounds and with non-metals to form covalent compounds.
- Diatomic molecules: They exist as diatomic molecules (e.g., F₂, Cl₂, Br₂, I₂).
- Variable states of matter: Fluorine and chlorine are gases at room temperature, bromine is a liquid, and iodine is a solid.
Applications of Halogens:
- Fluorine: Used in the production of fluorocarbons (refrigerants, plastics) and in toothpaste (fluoride).
- Chlorine: Used as a disinfectant in water treatment and in the production of various chemicals, including PVC plastic.
- Bromine: Used as a flame retardant and in the production of photographic chemicals.
- Iodine: Essential nutrient for human health and used as a disinfectant and in various chemical processes.
The Noble Gases (Group 18)
The noble gases – helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) – are inert monatomic gases found in Group 18. They possess a complete valence shell of eight electrons (except helium with two), making them extremely unreactive.
Key Properties of Noble Gases:
- Low reactivity: They are extremely unreactive due to their stable electron configurations.
- Monatomic gases: They exist as monatomic gases at room temperature.
- Colorless, odorless, and tasteless: They are generally colorless, odorless, and tasteless.
Applications of Noble Gases:
- Helium: Used in balloons, cryogenics, and MRI machines due to its low density and inertness.
- Neon: Used in neon signs due to its bright red-orange glow when electrically excited.
- Argon: Used as an inert atmosphere in welding and in incandescent light bulbs.
- Krypton and Xenon: Used in specialized lighting applications.
Beyond the Main Groups: Lanthanides and Actinides
The lanthanides (rare earth elements) and actinides are two series of elements placed separately at the bottom of the periodic table. They are characterized by the filling of the 4f and 5f orbitals, respectively, resulting in similar chemical properties within each series. Many are radioactive.
Lanthanides:
Used in various high-tech applications, including magnets, lasers, and catalysts.
Actinides:
Mostly radioactive, with several elements finding applications in nuclear reactors and weapons.
Conclusion: The Importance of Chemical Families
Understanding the chemical families within the periodic table is crucial for predicting the behavior of elements and compounds. The systematic organization based on electron configuration allows chemists to anticipate reactivity, bonding patterns, and physical properties. This knowledge is essential for designing new materials, developing novel chemical processes, and understanding the role of elements in biological systems and the environment. Further exploration into the specific properties of individual elements within each family reveals the rich diversity and intricate relationships within the periodic table, showcasing its enduring value as a fundamental tool in chemistry. Continued research into the properties and applications of these chemical families promises further breakthroughs in science and technology.
Latest Posts
Latest Posts
-
Is Urea The Same As Uric Acid
Mar 31, 2025
-
How To Determine The Highest Boiling Point
Mar 31, 2025
-
How To Explain 10x In Lab Math
Mar 31, 2025
-
How To Make A Normal Probability Plot
Mar 31, 2025
-
Introspection Refers To A Process By Which Someone Examines
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Chemical Families In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
