Classifying Chemical Reactions Worksheet With Answers
Muz Play
Apr 07, 2025 · 5 min read
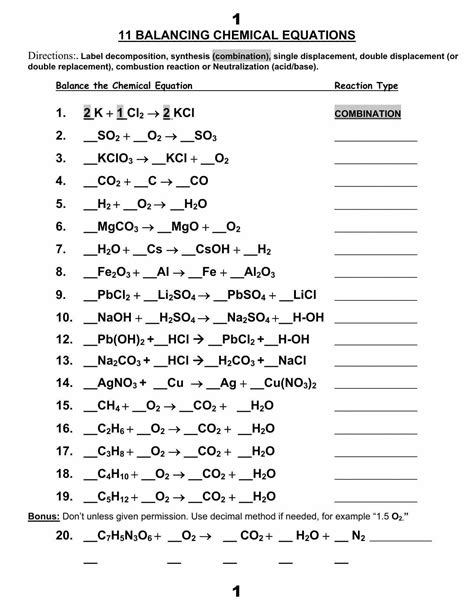
Table of Contents
Classifying Chemical Reactions Worksheet: A Comprehensive Guide with Answers
This worksheet is designed to help you master the art of classifying chemical reactions. Understanding the different types of chemical reactions is fundamental to comprehending chemistry. This guide will walk you through various reaction types, providing examples and explanations to solidify your understanding. We’ll also provide a practice worksheet with detailed answers to test your knowledge.
Types of Chemical Reactions
Chemical reactions are processes that lead to the transformation of one or more substances into new substances with different properties. These transformations can be categorized into several types, each characterized by specific features.
1. Synthesis (Combination) Reactions
In a synthesis reaction, two or more substances combine to form a more complex product. The general form is:
A + B → AB
Examples:
- Formation of water: 2H₂ + O₂ → 2H₂O
- Formation of magnesium oxide: 2Mg + O₂ → 2MgO
- Formation of sodium chloride: 2Na + Cl₂ → 2NaCl
Key Characteristics: A single product is formed from two or more reactants. These reactions often involve the combination of elements to form compounds.
2. Decomposition Reactions
Decomposition reactions are the opposite of synthesis reactions. A single compound breaks down into two or more simpler substances. The general form is:
AB → A + B
Examples:
- Decomposition of water: 2H₂O → 2H₂ + O₂
- Decomposition of calcium carbonate: CaCO₃ → CaO + CO₂
- Electrolysis of water: 2H₂O → 2H₂ + O₂
Key Characteristics: A single reactant breaks down into two or more products. Often requires energy input, such as heat or electricity.
3. Single Displacement (Replacement) Reactions
In a single displacement reaction, a more reactive element replaces a less reactive element in a compound. The general form is:
A + BC → AC + B
Examples:
- Zinc reacting with hydrochloric acid: Zn + 2HCl → ZnCl₂ + H₂
- Iron reacting with copper(II) sulfate: Fe + CuSO₄ → FeSO₄ + Cu
- Magnesium reacting with water: Mg + 2H₂O → Mg(OH)₂ + H₂
Key Characteristics: One element replaces another in a compound. Reactivity series helps predict the outcome.
4. Double Displacement (Metathesis) Reactions
Double displacement reactions involve the exchange of ions between two compounds. The general form is:
AB + CD → AD + CB
Examples:
- Precipitation reaction: AgNO₃ + NaCl → AgCl + NaNO₃ (AgCl is a precipitate)
- Neutralization reaction: HCl + NaOH → NaCl + H₂O
- Gas-forming reaction: Na₂CO₃ + 2HCl → 2NaCl + H₂O + CO₂
Key Characteristics: Ions exchange partners; often involves the formation of a precipitate, a gas, or water.
5. Combustion Reactions
Combustion reactions involve the rapid reaction of a substance with oxygen, usually producing heat and light. The general form is:
Fuel + O₂ → Products (usually CO₂ and H₂O)
Examples:
- Burning of methane: CH₄ + 2O₂ → CO₂ + 2H₂O
- Burning of propane: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
- Burning of ethanol: C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O
Key Characteristics: Involves rapid oxidation; produces heat and light; often involves hydrocarbons.
Classifying Chemical Reactions Worksheet
Now, let’s put your knowledge to the test! Classify the following chemical reactions, providing a brief justification for your answer.
Instructions: Identify the type of reaction (synthesis, decomposition, single displacement, double displacement, or combustion) for each of the following chemical equations.
1. 2KClO₃ → 2KCl + 3O₂
2. Fe + CuSO₄ → FeSO₄ + Cu
3. 2Na + Cl₂ → 2NaCl
4. Ca(OH)₂ + 2HCl → CaCl₂ + 2H₂O
5. C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
6. Zn + 2HCl → ZnCl₂ + H₂
7. 2HgO → 2Hg + O₂
8. AgNO₃ + NaCl → AgCl + NaNO₃
9. Mg + 2H₂O → Mg(OH)₂ + H₂
10. 2Al + 3Cl₂ → 2AlCl₃
Answers and Explanations
Let's review the answers and delve deeper into the reasoning behind the classifications.
1. 2KClO₃ → 2KCl + 3O₂ --- Decomposition: A single compound (KClO₃) breaks down into two simpler substances (KCl and O₂). This is a classic example of a decomposition reaction.
2. Fe + CuSO₄ → FeSO₄ + Cu --- Single Displacement: Iron (Fe) replaces copper (Cu) in copper(II) sulfate (CuSO₄). This is a single displacement reaction based on the relative reactivity of iron and copper.
3. 2Na + Cl₂ → 2NaCl --- Synthesis: Two elements (sodium and chlorine) combine to form a single compound (sodium chloride). This is a straightforward synthesis reaction.
4. Ca(OH)₂ + 2HCl → CaCl₂ + 2H₂O --- Double Displacement: Calcium hydroxide and hydrochloric acid exchange ions to form calcium chloride and water. This is a neutralization reaction, a specific type of double displacement reaction.
5. C₃H₈ + 5O₂ → 3CO₂ + 4H₂O --- Combustion: Propane (C₃H₈) reacts with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O), releasing energy in the process. This is a combustion reaction.
6. Zn + 2HCl → ZnCl₂ + H₂ --- Single Displacement: Zinc (Zn) replaces hydrogen (H) in hydrochloric acid (HCl). This is another example of a single displacement reaction.
7. 2HgO → 2Hg + O₂ --- Decomposition: Mercury(II) oxide (HgO) decomposes into mercury (Hg) and oxygen (O₂). This is a thermal decomposition reaction.
8. AgNO₃ + NaCl → AgCl + NaNO₃ --- Double Displacement: Silver nitrate and sodium chloride exchange ions to form silver chloride (a precipitate) and sodium nitrate. This is a precipitation reaction, a subtype of double displacement.
9. Mg + 2H₂O → Mg(OH)₂ + H₂ --- Single Displacement: Magnesium (Mg) reacts with water to displace hydrogen (H₂) and form magnesium hydroxide.
10. 2Al + 3Cl₂ → 2AlCl₃ --- Synthesis: Two elements (aluminum and chlorine) combine to form a single compound (aluminum chloride). This is a direct combination or synthesis reaction.
Further Practice and Resources
To further solidify your understanding, try working through additional practice problems from your textbook or online resources. Focus on understanding the underlying principles of each reaction type and the driving forces behind them. Consider researching the reactivity series of metals to help predict the outcomes of single displacement reactions. Mastering the classification of chemical reactions is a crucial stepping stone to understanding more complex chemical concepts. Remember, practice makes perfect! Keep practicing and you’ll become an expert in classifying chemical reactions.
Latest Posts
Latest Posts
-
Who Are Users Of Accounting Information
Apr 10, 2025
-
Complex Zeros Of A Polynomial Function
Apr 10, 2025
-
Is Mmol L The Same As Meq L
Apr 10, 2025
-
Does Protists Reproduce Sexually Or Asexually
Apr 10, 2025
-
A Non Profit Organization Plans To Hold A Raffle
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about Classifying Chemical Reactions Worksheet With Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.