Columns Of The Periodic Table Are Called
Muz Play
Mar 15, 2025 · 6 min read
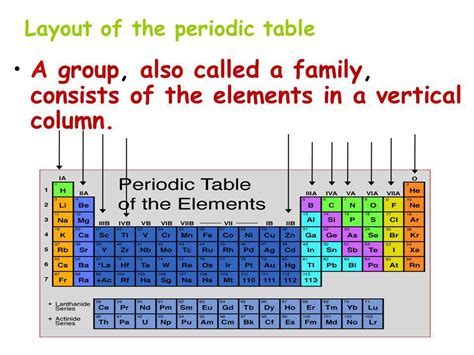
Table of Contents
Columns of the Periodic Table are Called Groups or Families: A Deep Dive into Chemical Organization
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its layout is crucial for grasping the fundamental principles of chemistry. One of the most basic, yet vital, concepts is understanding that the columns of the periodic table are called groups or families. This article will delve deep into the significance of these groups, exploring their properties, trends, and the underlying reasons for their similarities.
Understanding the Organization of the Periodic Table
The periodic table isn't just a random arrangement of elements; it's a carefully crafted system reflecting the periodic recurrence of chemical and physical properties. Elements are arranged in increasing order of their atomic number (number of protons), which dictates their chemical behavior. The arrangement isn't arbitrary; it reveals profound relationships between elements.
The table is composed of:
-
Rows (Periods): These horizontal rows represent elements with the same number of electron shells. As you move across a period, the number of electrons in the outermost shell (valence electrons) increases, leading to changes in properties.
-
Columns (Groups or Families): These vertical columns are the focus of this article. Elements within the same group share similar chemical properties due to having the same number of valence electrons. This is a crucial aspect of chemical bonding and reactivity.
Why are Columns Called Groups and Families?
The terms "groups" and "families" are used interchangeably to refer to the columns of the periodic table. Both terms highlight the similarities and shared characteristics of the elements within a column:
-
Groups: This term emphasizes the collective nature of elements within a column. They are grouped together because they exhibit similar behavior.
-
Families: This term evokes a sense of kinship or relatedness. Elements in the same family share a common ancestry in terms of their electron configuration and, consequently, their chemical properties.
Understanding this terminology is essential for comprehending the periodic trends and predicting the behavior of elements.
The Significance of Valence Electrons in Group Properties
The reason elements within the same group exhibit similar properties boils down to their valence electrons. These are the electrons in the outermost electron shell. They are the ones primarily involved in chemical bonding and interactions. Elements in the same group have the same number of valence electrons, leading to predictable chemical behavior.
For instance, elements in Group 1 (alkali metals) all have one valence electron. This single valence electron readily participates in chemical reactions, resulting in similar reactivity patterns across the group. They are all highly reactive, readily forming +1 ions, and react vigorously with water.
Similarly, elements in Group 18 (noble gases) have a full valence shell (eight electrons, except for Helium with two). This complete shell makes them exceptionally stable and unreactive, explaining their inert nature.
Exploring Key Groups and Their Characteristics
Let's examine some prominent groups and their defining features:
Group 1: Alkali Metals
- Characteristics: Highly reactive metals, soft, low density, low melting points, readily lose one electron to form +1 ions.
- Examples: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), Francium (Fr).
- Reactivity Trends: Reactivity increases down the group as the outermost electron becomes further from the nucleus and easier to lose.
Group 2: Alkaline Earth Metals
- Characteristics: Reactive metals, harder and denser than alkali metals, higher melting points, readily lose two electrons to form +2 ions.
- Examples: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
- Reactivity Trends: Reactivity increases down the group, similar to alkali metals.
Group 17: Halogens
- Characteristics: Highly reactive nonmetals, exist as diatomic molecules (e.g., Cl₂), readily gain one electron to form -1 ions, highly electronegative.
- Examples: Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), Astatine (At).
- Reactivity Trends: Reactivity decreases down the group as the added electron is further from the nucleus and less attracted.
Group 18: Noble Gases
- Characteristics: Inert gases, extremely unreactive due to their complete valence shells, exist as monatomic gases.
- Examples: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn).
- Reactivity Trends: Generally unreactive, although some heavier noble gases can form compounds under specific conditions.
Transition Metals: A Unique Group
The transition metals occupy the central block of the periodic table. They are characterized by:
- Variable Oxidation States: They can form ions with different charges, leading to a wide range of compounds.
- Colored Compounds: Many transition metal compounds exhibit vibrant colors due to the absorption and emission of light by d-electrons.
- Catalytic Properties: Many transition metals and their compounds act as catalysts in various chemical reactions.
- Magnetic Properties: Some transition metals exhibit magnetic properties due to unpaired electrons.
The transition metals show less consistent trends in properties compared to the main group elements, reflecting the complexity of their electronic configurations.
Lanthanides and Actinides: The Inner Transition Metals
Located at the bottom of the periodic table, these two series are also known as inner transition metals. They are characterized by:
- f-block Elements: They are filling their f-subshells, leading to unique chemical properties.
- Similar Chemical Properties: Elements within each series have very similar properties due to the gradual filling of the f-orbitals.
- Radioactivity: Many actinides are radioactive, contributing to their importance in nuclear chemistry.
Periodic Trends within Groups
As you move down a group, certain trends in properties are observed:
- Atomic Radius: Increases down a group due to the addition of electron shells.
- Electronegativity: Generally decreases down a group as the outermost electrons are further from the nucleus and less strongly attracted.
- Ionization Energy: Generally decreases down a group for the same reason.
- Metallic Character: Generally increases down a group as the outermost electrons are more easily lost.
Applications and Importance of Group Understanding
Understanding the groups of the periodic table is essential in various fields:
- Predicting Chemical Reactions: Knowing the group of an element allows chemists to predict its reactivity and how it will behave in different chemical reactions.
- Designing New Materials: The properties of elements within groups are crucial in designing new materials with specific characteristics.
- Developing New Technologies: The understanding of group properties drives advancements in various technologies, including electronics, medicine, and energy.
- Environmental Science: Understanding the behavior of elements in the environment, particularly their reactivity and toxicity, relies heavily on knowledge of their group properties.
Conclusion: The Power of Organization
The columns of the periodic table, known as groups or families, are not merely a convenient organizational tool; they represent a fundamental understanding of the underlying principles of chemical behavior. The shared properties within each group stem from the consistent number of valence electrons, providing a powerful framework for predicting and understanding the reactivity and characteristics of elements. From predicting chemical reactions to designing advanced materials, the understanding of groups and families is paramount to advancing our knowledge of chemistry and its applications. Mastering this concept is fundamental to success in chemistry and related scientific fields. The organized structure of the periodic table, with its clearly defined groups and periods, serves as a testament to the power of organization in unraveling the complexities of the natural world.
Latest Posts
Latest Posts
-
A Chemical Reaction Is Balanced By Changing The
May 09, 2025
-
First Law Of Thermodynamics Open System
May 09, 2025
-
Which Process Is Used To Produce Beer And Wine
May 09, 2025
-
Can Homogeneous Mixtures Be Separated By Physical Means
May 09, 2025
-
How Many Grams Is A Water Bottle
May 09, 2025
Related Post
Thank you for visiting our website which covers about Columns Of The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.