Compare And Contrast Hydrogen Bonds With Van Der Waals Interactions
Muz Play
Mar 15, 2025 · 6 min read
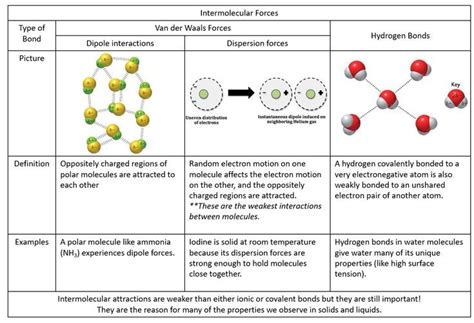
Table of Contents
Hydrogen Bonds vs. Van der Waals Interactions: A Detailed Comparison
Understanding intermolecular forces is crucial for comprehending the properties of matter. Among these forces, hydrogen bonds and van der Waals interactions are particularly significant, influencing everything from the structure of proteins to the boiling points of liquids. While both contribute to the overall attraction between molecules, they differ significantly in strength and mechanism. This article provides a detailed comparison and contrast of hydrogen bonds and van der Waals interactions, highlighting their similarities, differences, and the critical roles they play in various systems.
Defining the Forces: Hydrogen Bonds and Van der Waals Interactions
Let's start by clearly defining each type of intermolecular force.
Hydrogen Bonds: A Special Type of Dipole-Dipole Interaction
A hydrogen bond is a special type of dipole-dipole interaction that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule (or even a different part of the same molecule). This electronegativity difference creates a significant dipole moment, where the hydrogen atom carries a partial positive charge (δ+) and the electronegative atom carries a partial negative charge (δ-). The strong electrostatic attraction between these opposite charges constitutes the hydrogen bond. It's important to note that hydrogen bonds are stronger than typical dipole-dipole interactions.
Key Characteristics of Hydrogen Bonds:
- Stronger than typical dipole-dipole interactions: The relatively large difference in electronegativity between hydrogen and the electronegative atom leads to a strong electrostatic attraction.
- Highly directional: Hydrogen bonds are directional, meaning they tend to form along a straight line between the hydrogen atom and the electronegative atom. This directionality significantly affects molecular structure and properties.
- Involvement of electronegative atoms: The presence of highly electronegative atoms (O, N, F) is essential for hydrogen bond formation.
- Influence on physical properties: Hydrogen bonds have a profound impact on the boiling points, melting points, and viscosities of substances. For example, water's unusually high boiling point is directly attributed to the extensive hydrogen bonding network between water molecules.
Van der Waals Interactions: A Collective Term
Van der Waals interactions are a broader category of weak intermolecular forces encompassing several types of attractions:
- London Dispersion Forces (LDFs): These are the weakest type of van der Waals interaction and arise from temporary, instantaneous dipoles that occur due to the fluctuating electron distribution within molecules. Even nonpolar molecules experience LDFs. The larger the molecule (and thus the larger its electron cloud), the stronger the LDFs.
- Dipole-Dipole Interactions: These occur between polar molecules, where permanent dipoles attract each other. They are stronger than LDFs but weaker than hydrogen bonds.
- Ion-Dipole Interactions: These involve the interaction between an ion (either positive or negative) and a polar molecule. The strength of this interaction depends on the charge of the ion and the polarity of the molecule.
Key Characteristics of Van der Waals Interactions:
- Weaker than hydrogen bonds: Van der Waals forces are significantly weaker than hydrogen bonds.
- Additive effect: While individually weak, the cumulative effect of numerous van der Waals interactions can be substantial, especially in large molecules.
- Present in all molecules: All molecules, regardless of polarity, experience van der Waals interactions. LDFs are always present.
- Influence on physical properties: Van der Waals forces influence the physical properties of substances, but their effect is generally less dramatic than that of hydrogen bonds.
Comparing and Contrasting Hydrogen Bonds and Van der Waals Interactions
The following table summarizes the key differences and similarities between hydrogen bonds and van der Waals interactions:
| Feature | Hydrogen Bonds | Van der Waals Interactions |
|---|---|---|
| Strength | Strongest type of intermolecular force | Weakest types of intermolecular forces |
| Type of Force | Special type of dipole-dipole interaction | Includes LDFs, dipole-dipole, and ion-dipole |
| Requirement | Highly electronegative atoms (O, N, F) bonded to H | No specific atom requirements; present in all molecules |
| Directionality | Highly directional | Generally non-directional (except dipole-dipole) |
| Influence on Properties | Significant impact on boiling points, melting points, etc. | Less significant impact than hydrogen bonds |
| Examples | Water (H₂O), DNA, proteins | Noble gases, nonpolar hydrocarbons, many organic molecules |
The Significance of These Forces in Biological Systems
Both hydrogen bonds and van der Waals interactions are essential for the structure and function of biological molecules.
Hydrogen Bonds in Biology
Hydrogen bonds are crucial for:
- Protein Structure: Hydrogen bonds stabilize the secondary (alpha-helices and beta-sheets) and tertiary structures of proteins. The specific arrangement of hydrogen bonds dictates the protein's three-dimensional shape, which is vital for its function.
- DNA Structure: The double helix structure of DNA is maintained by hydrogen bonds between complementary base pairs (adenine with thymine, and guanine with cytosine). These bonds allow for the precise replication and transcription of genetic information.
- Enzyme-Substrate Interactions: Hydrogen bonds play a role in the binding of substrates to enzymes, facilitating catalytic activity.
- Water's Unique Properties: The extensive hydrogen bonding network in water contributes to its high surface tension, high boiling point, and its ability to act as a universal solvent, all essential for life.
Van der Waals Interactions in Biology
Despite being weaker, van der Waals interactions are collectively significant in biological systems:
- Protein Folding: Although weaker than hydrogen bonds, the cumulative effect of numerous van der Waals interactions helps to stabilize the overall tertiary structure of proteins. They contribute to the precise packing of amino acid residues within the protein.
- Enzyme-Substrate Interactions: Van der Waals interactions contribute to the binding affinity between enzymes and their substrates.
- Membrane Interactions: Van der Waals interactions between lipid molecules help maintain the integrity of cell membranes.
- Molecular Recognition: Weak interactions, including van der Waals forces, play a critical role in molecular recognition processes, essential for various biological interactions.
Beyond Biology: Applications in Materials Science and Beyond
The understanding of hydrogen bonds and van der Waals interactions extends far beyond biology. These forces significantly influence the properties of many materials:
- Polymers: The strength and flexibility of polymers are influenced by the types and strengths of intermolecular forces present, including hydrogen bonds and van der Waals interactions. Stronger intermolecular forces typically result in stronger and more rigid polymers.
- Crystals: The packing arrangement of molecules in crystals is determined by the interplay of various intermolecular forces, including van der Waals interactions and, in some cases, hydrogen bonds.
- Liquids and Gases: The boiling points and vapor pressures of liquids and gases are directly related to the strength of intermolecular forces, with stronger forces leading to higher boiling points and lower vapor pressures.
- Drug Design: Understanding intermolecular forces is crucial in drug design, as the binding of drugs to their target molecules relies heavily on these interactions.
Conclusion
Hydrogen bonds and van der Waals interactions are both crucial intermolecular forces influencing the structure and properties of various substances. While hydrogen bonds are stronger and more directional, van der Waals interactions are ubiquitous and their collective effect can be substantial. The interplay between these forces dictates the behavior of molecules in various contexts, from biological systems to materials science applications. A deep understanding of their differences and similarities is essential for advancements in diverse scientific fields. Further research continues to reveal the intricate roles of these forces in a wide range of phenomena, shaping our understanding of the world around us at a molecular level.
Latest Posts
Latest Posts
-
The Library Is An Example Of What Type Of Resource
May 09, 2025
-
The Principal Force Driving Movement In Diffusion Is
May 09, 2025
-
What Part Of The Cell Stores Material Within The Cell
May 09, 2025
-
How To Write An Equation For A Function
May 09, 2025
-
Why Does Soap Weaken Hydrogen Bonds
May 09, 2025
Related Post
Thank you for visiting our website which covers about Compare And Contrast Hydrogen Bonds With Van Der Waals Interactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.