Compare The Mass Of An Electron To A Proton
Muz Play
Mar 17, 2025 · 6 min read
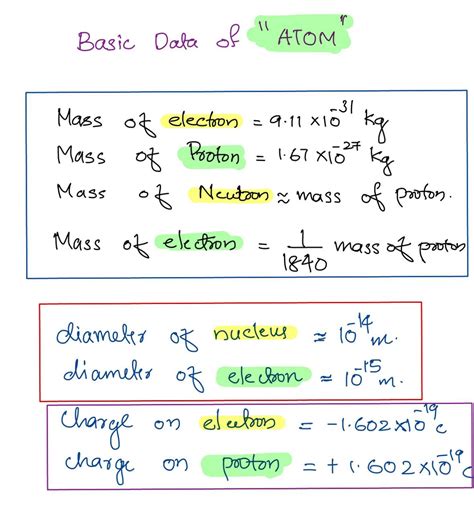
Table of Contents
Comparing the Mass of an Electron to a Proton: A Deep Dive into Subatomic Particles
The atom, once considered the fundamental building block of matter, is now understood to be a complex system composed of even smaller particles. Among these, the electron and the proton stand out as key players, defining the atom's electrical charge and much of its mass. While both are crucial for atomic structure and behavior, their masses differ dramatically. This article will delve deep into the comparison of electron and proton masses, exploring their relative sizes, the implications of this mass difference, and the methods used to measure these incredibly tiny quantities.
The Astonishing Mass Discrepancy: Electrons are Featherlight Compared to Protons
The most striking difference between an electron and a proton lies in their masses. A proton is approximately 1836 times more massive than an electron. This is a colossal difference on a subatomic scale. To visualize this, imagine a proton as a bowling ball; an electron would then be a tiny marble, barely registering on the scale compared to the bowling ball's weight.
This massive disparity has profound implications for atomic behavior and the properties of matter as a whole. The relatively negligible mass of the electron contributes to its wave-like behavior, as described by quantum mechanics, while the proton's much larger mass anchors the atom's nucleus.
Numerical Values and Units
To be precise, the mass of:
-
An electron (mₑ): 9.109 x 10⁻³¹ kilograms (kg) or 0.511 MeV/c² (using Einstein's mass-energy equivalence)
-
A proton (mₚ): 1.673 x 10⁻²⁷ kilograms (kg) or 938.3 MeV/c²
The use of MeV/c² (mega-electronvolts per speed of light squared) is common in particle physics because it directly relates mass to energy. This unit reflects the energy required to create a particle from pure energy, as described by Einstein's famous equation, E=mc².
Methods for Measuring Subatomic Masses
Determining the masses of subatomic particles like electrons and protons is no easy feat. These particles are incredibly tiny, requiring sophisticated techniques and advanced instrumentation. Here are some of the primary methods employed:
1. Mass Spectrometry
Mass spectrometry is a widely used technique that measures the mass-to-charge ratio (m/z) of ions. By accelerating ions in an electric or magnetic field and measuring their deflection, scientists can determine their mass-to-charge ratio. While not directly measuring mass, it provides a relative measure that, when combined with knowledge of charge, allows the calculation of mass. Different types of mass spectrometers exist, each optimized for different mass ranges and sensitivities.
2. Magnetic Resonance Techniques
Nuclear Magnetic Resonance (NMR) and Electron Paramagnetic Resonance (EPR) are spectroscopic techniques that exploit the magnetic properties of atomic nuclei and unpaired electrons, respectively. By analyzing the resonant frequencies of these particles in a magnetic field, scientists can deduce information related to their mass and other properties. While not directly measuring mass, these techniques provide indirect measurements that are crucial for understanding the interaction between mass and other fundamental properties.
3. Particle Accelerators and Collider Experiments
Particle accelerators, like the Large Hadron Collider (LHC), are incredibly powerful machines that accelerate charged particles to near-light speeds and then collide them. By analyzing the resulting debris, scientists can study the properties of fundamental particles, including their masses. These collisions produce a multitude of particles, and sophisticated detectors measure their trajectories and energies, enabling the calculation of their masses using conservation laws and relativistic equations.
The Implications of the Mass Difference
The significant mass difference between electrons and protons has profound consequences for the structure and behavior of atoms and molecules:
-
Atomic Nucleus Stability: The vastly larger mass of the proton makes it the central component of the atomic nucleus. The protons, along with neutrons (which have a slightly larger mass than protons), form the dense, positively charged core of the atom, which contains almost all of the atom's mass. The relatively light electrons orbit this nucleus.
-
Chemical Bonding: The electrons, with their much smaller mass and ability to move more freely, play the dominant role in chemical bonding. They are the particles involved in the formation of chemical bonds, dictating the interactions between atoms and molecules, thereby shaping the properties of materials. The proton's mass primarily affects the overall mass of the atom and its gravitational interactions, but not directly involved in chemical bonding mechanisms.
-
Isotopes: The number of protons determines an element's identity (its atomic number), while the number of neutrons determines its isotopic form. Isotopes of the same element have the same number of protons but differing numbers of neutrons, resulting in variations in atomic mass while maintaining the same chemical behavior. This diversity in isotopes is a direct result of the fact that adding neutrons (slightly heavier than protons) doesn't fundamentally alter the atom's chemical properties.
-
Nuclear Reactions: The mass difference between protons and electrons is crucial in understanding nuclear reactions like nuclear fusion and fission. These processes involve significant changes in the number of protons and neutrons within the nucleus, releasing or absorbing immense amounts of energy, according to Einstein's mass-energy equivalence.
Beyond Mass: Other Key Differences
While the mass difference is the most prominent, other significant differences exist between electrons and protons:
-
Charge: Electrons carry a single negative charge (-1e), while protons carry a single positive charge (+1e), where 'e' is the elementary charge. This opposite charge is responsible for the electrostatic attraction that binds electrons to the nucleus, forming a stable atom.
-
Spin: Both electrons and protons possess an intrinsic angular momentum called spin, quantized in units of ħ/2 (reduced Planck constant). This spin contributes to their magnetic properties and plays a significant role in atomic and molecular interactions.
-
Composition: Electrons are considered fundamental particles – elementary particles of the Standard Model of particle physics, meaning they are not made up of smaller constituents. Protons, on the other hand, are composite particles, made up of three quarks (two up quarks and one down quark) held together by the strong nuclear force mediated by gluons.
-
Location within the Atom: Electrons occupy the space surrounding the nucleus in atomic orbitals, while protons reside within the nucleus itself.
Conclusion: A Fundamental Disparity with Profound Consequences
The vast difference in mass between an electron and a proton is not merely a numerical curiosity. It's a fundamental aspect of atomic structure that underpins the properties of matter, shaping the interactions between atoms, molecules, and even nuclei. The relatively negligible mass of the electron allows it to exhibit wave-like behavior and participate in chemical bonding, while the much larger mass of the proton anchors the atomic nucleus, determining its stability and overall mass. Understanding this disparity is crucial to comprehending the behavior of matter at its most fundamental level, and it remains a cornerstone of our understanding of the physical world. Further research into the properties of these fundamental particles continues to refine our models and reveal more about the intricate workings of the universe.
Latest Posts
Latest Posts
-
Can Mitochondria Survive Outside The Cell
Mar 17, 2025
-
Name A Structural Difference Between Triglycerides And Phospholipids
Mar 17, 2025
-
Ending Materials In A Chemical Reaction
Mar 17, 2025
-
Get Energy By Eating Other Organisms
Mar 17, 2025
-
What Is A Power Function In Math
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Compare The Mass Of An Electron To A Proton . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
