Degree Of Unsaturation Formula With Oxygen
Muz Play
Mar 17, 2025 · 5 min read
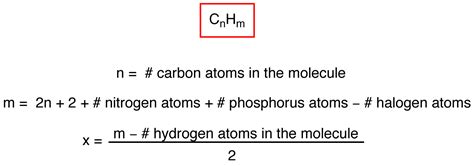
Table of Contents
Degree of Unsaturation Formula with Oxygen: A Comprehensive Guide
The degree of unsaturation, also known as the index of hydrogen deficiency (IHD), is a crucial concept in organic chemistry. It helps us determine the number of rings and pi bonds present in a molecule, providing valuable insights into its structure. While the basic formula is well-known, incorporating oxygen atoms requires careful consideration. This comprehensive guide will delve into the degree of unsaturation formula, specifically addressing the nuances of incorporating oxygen. We'll explore the formula's derivation, applications, and limitations, providing a thorough understanding of this essential tool for organic chemists.
Understanding the Basic Degree of Unsaturation Formula
Before incorporating oxygen, let's establish the fundamental degree of unsaturation formula for hydrocarbon molecules (containing only carbon and hydrogen):
IHD = (2C + 2 + N - X - H) / 2
Where:
- C represents the number of carbon atoms.
- N represents the number of nitrogen atoms.
- X represents the number of halogen atoms (F, Cl, Br, I).
- H represents the number of hydrogen atoms.
This formula essentially compares the number of hydrogen atoms in a saturated hydrocarbon (alkanes) to the actual number of hydrogen atoms in the molecule. Each unsaturation (a double bond, triple bond, or ring) reduces the number of hydrogen atoms by two. Therefore, dividing the difference by two gives the degree of unsaturation.
Example: Calculating IHD for a Hydrocarbon
Let's calculate the IHD for cyclohexene (C₆H₁₀):
C = 6, H = 10, N = 0, X = 0
IHD = (2 * 6 + 2 + 0 - 0 - 10) / 2 = 2
This result indicates that cyclohexene has two degrees of unsaturation: one from the ring and one from the double bond.
Incorporating Oxygen into the Degree of Unsaturation Formula
Oxygen atoms, being divalent (forming two bonds), do not affect the degree of unsaturation. They do not alter the overall hydrogen deficiency in the molecule. This is because the two bonds an oxygen atom forms essentially replace two hydrogen atoms in a saturated framework without changing the total number of pi bonds or rings.
Therefore, when calculating the IHD for molecules containing oxygen, we can simply ignore the oxygen atoms and use the same formula as before:
IHD = (2C + 2 + N - X - H) / 2
Example: Calculating IHD for a Molecule Containing Oxygen
Let's calculate the IHD for acetone (C₃H₆O):
C = 3, H = 6, N = 0, X = 0, O = 1 (ignore O for the calculation)
IHD = (2 * 3 + 2 + 0 - 0 - 6) / 2 = 1
Acetone has one degree of unsaturation, which corresponds to the carbonyl (C=O) double bond. Notice how the oxygen atom didn't affect the calculation.
Dealing with Other Heteroatoms: Nitrogen and Halogens
While oxygen's presence is straightforward, other heteroatoms require adjustments to the formula.
-
Nitrogen: Each nitrogen atom adds one to the IHD because it contributes one more bond than a hydrogen atom (it forms three bonds, while hydrogen forms one).
-
Halogens: Halogens (F, Cl, Br, I) are treated as equivalent to hydrogen atoms. Each halogen atom contributes one to the hydrogen count.
Example: Calculating IHD with Nitrogen and Halogens
Let's calculate the IHD for chlorobenzene (C₆H₅Cl):
C = 6, H = 5, N = 0, X = 1 (Cl)
IHD = (2 * 6 + 2 + 0 - 1 - 5) / 2 = 4
Chlorobenzene has four degrees of unsaturation: one from the ring and three from the three double bonds in the benzene ring.
Advanced Applications and Considerations
The degree of unsaturation formula is a powerful tool, but its application requires careful consideration.
-
Isomers: Molecules with the same molecular formula but different structures (isomers) can have the same IHD. The IHD alone cannot distinguish between isomers. Further spectroscopic and chemical analysis is necessary.
-
Cumulative Unsaturations: The formula counts cumulative unsaturations (e.g., multiple double bonds or triple bonds in a conjugated system) as individual unsaturations.
-
Complex Molecules: For exceptionally complex molecules with numerous heteroatoms and structural features, detailed structural analysis and other spectroscopic techniques are crucial in confirming the structure.
-
Limitations: The IHD only provides the total number of pi bonds and rings; it doesn't specify their exact arrangement or type.
Practical Applications in Organic Chemistry
The degree of unsaturation is extensively used in organic chemistry for:
-
Structural Elucidation: Determining the possible structures of unknown compounds based on their molecular formula and spectral data. It narrows down the possibilities significantly before resorting to more sophisticated techniques.
-
Reaction Prediction: Predicting the outcome of reactions involving unsaturated compounds. Understanding the number of pi bonds and rings influences the reactivity of a molecule.
-
Synthesis Planning: Designing synthetic routes to complex molecules, understanding the number of unsaturations helps in strategically choosing reaction pathways.
-
Spectroscopic Interpretation: Complementing spectroscopic techniques like NMR and IR spectroscopy, the IHD provides a valuable piece of information for confirming or rejecting proposed structures.
Conclusion
The degree of unsaturation formula provides a simple yet powerful method for determining the number of pi bonds and rings in a molecule. While oxygen atoms do not affect the calculation directly, proper consideration of other heteroatoms such as nitrogen and halogens is crucial for accurate determination. By understanding the formula's derivation, applications, and limitations, organic chemists can effectively utilize it as a valuable tool for structural elucidation, reaction prediction, and synthesis planning. Always remember that while the IHD provides valuable information, it's just one piece of the puzzle; combining it with other spectroscopic and chemical data is essential for complete structural characterization of organic molecules. The more tools you have in your arsenal, the more confidently you can tackle the challenges presented by the intricate world of organic chemistry.
Latest Posts
Latest Posts
-
Can Mitochondria Survive Outside The Cell
Mar 17, 2025
-
Name A Structural Difference Between Triglycerides And Phospholipids
Mar 17, 2025
-
Ending Materials In A Chemical Reaction
Mar 17, 2025
-
Get Energy By Eating Other Organisms
Mar 17, 2025
-
What Is A Power Function In Math
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Degree Of Unsaturation Formula With Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
