Delta G Of A Carbonyl Reduction
Muz Play
Mar 18, 2025 · 6 min read
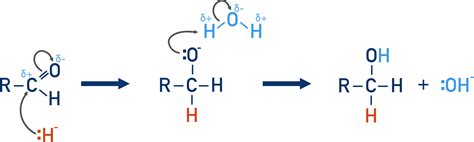
Table of Contents
Understanding the Delta G of a Carbonyl Reduction: A Deep Dive
The reduction of a carbonyl group, a fundamental reaction in organic chemistry, is a process rich in thermodynamic implications. Understanding the Gibbs Free Energy change (ΔG) associated with this transformation is crucial for predicting reaction spontaneity, equilibrium positions, and designing efficient synthetic strategies. This article will delve into the factors influencing the ΔG of carbonyl reduction, exploring various reduction methods and their associated thermodynamic profiles. We'll examine the role of sterics, electronics, and the nature of the reducing agent in shaping the overall free energy change.
The Thermodynamics of Carbonyl Reduction
The reduction of a carbonyl group, typically a ketone or aldehyde, involves the addition of two hydrogen atoms (or their equivalent) across the carbonyl double bond, converting it into an alcohol. This transformation can be represented generally as:
R1-C(=O)-R2 + 2H+ + 2e- ⇌ R1-CH(OH)-R2
The Gibbs Free Energy change (ΔG) for this reaction dictates its spontaneity. A negative ΔG indicates a spontaneous reaction (favoring product formation), while a positive ΔG indicates a non-spontaneous reaction (favoring reactants). A ΔG of zero signifies equilibrium. The ΔG is related to the equilibrium constant (K<sub>eq</sub>) through the following equation:
ΔG = -RTlnKeq
where R is the gas constant and T is the temperature in Kelvin.
Factors Affecting ΔG
Several factors contribute to the overall ΔG of a carbonyl reduction:
-
Nature of the carbonyl compound: The electronic and steric properties of the carbonyl compound significantly influence its reactivity and, consequently, the ΔG of reduction. Electron-withdrawing groups (EWGs) on the carbonyl carbon decrease electron density, making the carbonyl group less susceptible to nucleophilic attack (the first step in most reduction mechanisms). This results in a less negative (or potentially positive) ΔG. Conversely, electron-donating groups (EDGs) increase electron density, facilitating reduction and leading to a more negative ΔG. Steric hindrance around the carbonyl group can also impede approach of the reducing agent, increasing the activation energy and potentially affecting the overall ΔG.
-
Reducing agent: Different reducing agents possess varying reducing power, reflected in their respective standard reduction potentials (E°). Stronger reducing agents have more negative E° values, facilitating more exergonic reductions (more negative ΔG). Examples of common carbonyl reducing agents include lithium aluminum hydride (LiAlH<sub>4</sub>), sodium borohydride (NaBH<sub>4</sub>), and catalytic hydrogenation (H<sub>2</sub>/catalyst). Each agent offers a unique balance between reactivity, selectivity, and cost-effectiveness.
-
Solvent effects: The choice of solvent can influence the ΔG by affecting the solvation of reactants and products, as well as the stability of intermediates. Polar solvents generally stabilize charged intermediates, often lowering the activation energy and making the reaction more favorable.
-
Temperature: The temperature dependence of ΔG is expressed through the Gibbs-Helmholtz equation. Increasing temperature generally favors reactions with a positive ΔG, and this can be important for reactions where the equilibrium is unfavorable at lower temperatures.
Different Reduction Methods and Their ΔG Profiles
Let's examine the thermodynamic profiles of some common carbonyl reduction methods:
1. Lithium Aluminum Hydride (LiAlH₄) Reduction
LiAlH<sub>4</sub> is a powerful reducing agent capable of reducing a wide range of carbonyl compounds, including esters, carboxylic acids, and amides, in addition to ketones and aldehydes. Its high reactivity stems from the highly polarized Al-H bond, making it a strong nucleophile. This results in a significantly negative ΔG for the reduction of most carbonyl compounds. The reaction is typically carried out in anhydrous ether solvents to prevent decomposition of LiAlH<sub>4</sub>.
Thermodynamic Considerations: The highly negative ΔG reflects the strong driving force towards alcohol formation. The reaction is typically irreversible under standard conditions.
2. Sodium Borohydride (NaBH₄) Reduction
NaBH<sub>4</sub> is a milder reducing agent compared to LiAlH<sub>4</sub>. It preferentially reduces aldehydes and ketones, leaving esters and carboxylic acids unaffected. This selectivity is valuable in complex syntheses. The less negative ΔG compared to LiAlH<sub>4</sub> reflects its lower reducing power.
Thermodynamic Considerations: While still exergonic, the ΔG for NaBH<sub>4</sub> reduction is less negative than that of LiAlH<sub>4</sub>, reflecting a weaker driving force. The reaction is typically irreversible under standard conditions, although in some cases, the equilibrium can be manipulated.
3. Catalytic Hydrogenation
Catalytic hydrogenation involves the addition of hydrogen gas (H<sub>2</sub>) across the carbonyl double bond in the presence of a metal catalyst, such as palladium (Pd), platinum (Pt), or nickel (Ni). This method provides a highly selective and efficient reduction of aldehydes and ketones to their corresponding alcohols.
Thermodynamic Considerations: The ΔG for catalytic hydrogenation is generally negative, reflecting the thermodynamic favorability of the reaction. However, the precise value depends on the specific catalyst used, the solvent, and the reaction conditions. The reaction is often reversible under certain conditions, and the equilibrium position can be manipulated by changing pressure and temperature (Le Chatelier’s Principle).
4. Other Reduction Methods
Other methods for carbonyl reduction include using various metal hydrides (e.g., DIBAL-H), enzymatic reduction, and transfer hydrogenation. Each method possesses its own thermodynamic profile, influenced by the nature of the reducing agent and reaction conditions. The ΔG of these reactions can vary significantly, reflecting differences in reducing power and reaction mechanisms.
Predicting ΔG and its Significance in Synthesis
Precisely predicting the ΔG for a specific carbonyl reduction reaction is challenging, requiring sophisticated computational methods and detailed knowledge of the reaction mechanism and solvent effects. However, qualitative predictions can be made based on the factors discussed above.
Understanding the ΔG provides invaluable insights into reaction feasibility, equilibrium positions, and optimal reaction conditions. For instance, a highly negative ΔG indicates that the reaction will proceed readily and reach completion. A less negative or positive ΔG suggests a less favorable reaction, potentially requiring more stringent reaction conditions, such as elevated temperature or pressure, or the use of a stronger reducing agent to achieve a reasonable yield. In multi-step syntheses, knowledge of the ΔG for each step aids in optimizing the overall synthetic strategy. For reactions with small or positive ΔG values, the use of reaction coupling techniques to make the overall reaction exergonic is commonly employed.
Conclusion
The ΔG of a carbonyl reduction is a crucial thermodynamic parameter governing reaction spontaneity and equilibrium. This article has explored the multifaceted factors that influence ΔG, including the nature of the carbonyl compound, the reducing agent, solvent effects, and temperature. By understanding these factors and the thermodynamic profiles of different reduction methods, chemists can design efficient and selective synthetic strategies for carbonyl reduction, a cornerstone reaction in organic chemistry. Further exploration of this topic will likely include detailed computational analysis and advanced theoretical methods to predict ΔG with higher accuracy, allowing for more precise reaction design and optimization. The ongoing research in this field will continue to refine our understanding of this critical transformation.
Latest Posts
Latest Posts
-
T Test Formula For Dependent Samples
Mar 18, 2025
-
Eriksons Stage Of Integrity Vs Despair
Mar 18, 2025
-
What Is The Difference Between Microscopic And Macroscopic
Mar 18, 2025
-
Determine The Degrees Of Freedom For The F Statistic
Mar 18, 2025
-
Uniform Circular Motion Free Body Diagram
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Delta G Of A Carbonyl Reduction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
