Delta H Is Negative Exothermic Or Endothermic
Muz Play
Mar 31, 2025 · 6 min read
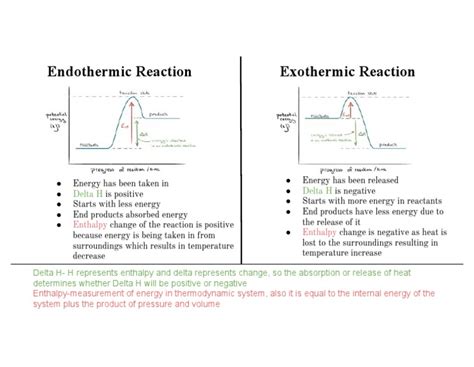
Table of Contents
Delta H is Negative: Exothermic or Endothermic? Understanding Enthalpy Change
Understanding enthalpy change (ΔH) is crucial in chemistry and thermodynamics. The sign of ΔH – positive or negative – directly indicates whether a reaction is exothermic or endothermic. This article delves deep into the meaning of a negative ΔH, clarifying its relationship to exothermic reactions, exploring real-world examples, and providing a comprehensive understanding of enthalpy changes in chemical and physical processes.
What is Enthalpy (H)?
Before tackling the significance of a negative ΔH, let's define enthalpy. Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. It's a state function, meaning its value depends only on the system's current state, not the path taken to reach that state. We can't directly measure enthalpy; instead, we measure changes in enthalpy (ΔH).
Understanding Enthalpy Change (ΔH)
The change in enthalpy (ΔH) reflects the heat transferred during a process at constant pressure. It's calculated as the difference between the enthalpy of the products and the enthalpy of the reactants:
ΔH = H<sub>products</sub> - H<sub>reactants</sub>
A negative ΔH indicates that the enthalpy of the products is lower than the enthalpy of the reactants. Conversely, a positive ΔH means the enthalpy of the products is higher than that of the reactants.
Negative ΔH: The Hallmark of Exothermic Reactions
A negative ΔH unequivocally signifies an exothermic reaction. In exothermic reactions, heat is released to the surroundings. The system loses energy, resulting in a decrease in its enthalpy. This energy release often manifests as an increase in temperature of the surroundings.
Think of it like this: the system is "losing" energy (heat) to become more stable, and this lost energy is transferred to the surroundings. This transfer is reflected in the negative value of ΔH.
Key Characteristics of Exothermic Reactions with Negative ΔH:
- Heat is released: The reaction produces heat, often causing a temperature increase in the immediate environment.
- ΔH is negative: This is the defining characteristic of an exothermic process.
- Products have lower enthalpy: The products are more stable than the reactants.
- Spontaneous (often, but not always): Many exothermic reactions proceed spontaneously, meaning they occur without external input of energy. However, spontaneity also depends on entropy (ΔS).
Examples of Exothermic Reactions (Negative ΔH)
Many everyday processes and chemical reactions are exothermic, featuring a negative ΔH. Here are a few striking examples:
1. Combustion Reactions:
The burning of fuels like wood, propane, or gasoline are classic examples of highly exothermic reactions. The reaction between fuel and oxygen releases significant heat, evident in the flames and warmth produced. The negative ΔH is substantial, making these reactions valuable energy sources.
2. Neutralization Reactions:
The reaction between an acid and a base is an exothermic process. When a strong acid, such as hydrochloric acid (HCl), reacts with a strong base, such as sodium hydroxide (NaOH), heat is released. This neutralization reaction generates water and a salt, accompanied by a noticeable temperature rise.
3. Formation of Water:
The formation of water from its constituent elements, hydrogen and oxygen, is highly exothermic. The reaction of hydrogen and oxygen gas generates significant heat and is used in some fuel cell technologies.
4. Respiration (Biological Exothermic Process):
The metabolic process of cellular respiration, which breaks down glucose to produce energy, is also exothermic. This process releases heat, maintaining our body temperature. While not as dramatically exothermic as combustion, it's crucial for life.
5. Condensation:
The phase transition from a gas to a liquid (condensation) is an exothermic process. When water vapor condenses into liquid water, heat is released into the surroundings. This is why condensation on a cold surface feels warm to the touch.
Differentiating Exothermic (Negative ΔH) from Endothermic (Positive ΔH)
It's critical to differentiate between exothermic (negative ΔH) and endothermic (positive ΔH) reactions. In endothermic reactions, heat is absorbed from the surroundings, increasing the system's enthalpy. The surroundings cool down as the system gains energy.
Here's a table summarizing the key differences:
| Feature | Exothermic (ΔH < 0) | Endothermic (ΔH > 0) |
|---|---|---|
| Heat Transfer | Heat released to surroundings | Heat absorbed from surroundings |
| ΔH Value | Negative | Positive |
| Temperature | Surrounding temperature increases | Surrounding temperature decreases |
| System Energy | Decreases | Increases |
| Product Stability | Products are more stable than reactants | Products are less stable than reactants |
| Examples | Combustion, neutralization, condensation | Melting ice, photosynthesis, boiling water |
Factors Affecting Enthalpy Change
Several factors influence the enthalpy change (ΔH) of a reaction:
- Nature of reactants and products: The chemical bonds and intermolecular forces in the reactants and products significantly impact ΔH. Stronger bonds in products lead to a more negative ΔH (exothermic).
- Reaction conditions (Temperature and Pressure): ΔH values are typically reported at standard temperature and pressure (STP). Changes in temperature and pressure can alter ΔH, though not always dramatically.
- Stoichiometry: The molar ratios of reactants and products influence the magnitude of ΔH.
Beyond Simple Reactions: Hess's Law and Enthalpy Calculations
For complex reactions that can't be directly measured, Hess's Law is incredibly useful. Hess's Law states that the total enthalpy change for a reaction is independent of the pathway taken. This means you can calculate the ΔH of a reaction by summing the ΔH values of a series of intermediate reactions that add up to the overall reaction.
This allows for the calculation of enthalpy changes for reactions that are difficult or impossible to measure directly in a laboratory setting.
Practical Applications of Understanding ΔH
Understanding enthalpy changes and the implications of a negative ΔH (exothermic reactions) has widespread practical applications, including:
- Energy production: Exothermic reactions are fundamental to generating energy from combustion of fuels.
- Industrial processes: Many industrial chemical processes rely on exothermic reactions to produce heat or drive other processes.
- Material science: Understanding enthalpy changes guides the development of new materials with desired properties.
- Environmental science: Enthalpy considerations are crucial for assessing the environmental impact of reactions and processes.
Conclusion: The Significance of a Negative ΔH
A negative ΔH definitively indicates an exothermic reaction, where heat is released to the surroundings, resulting in a decrease in the system's enthalpy. This fundamental concept is critical in chemistry, physics, biology, and engineering. Understanding exothermic reactions and their characteristic negative ΔH is crucial for predicting reaction behavior, designing processes, and solving problems across many scientific disciplines. The numerous examples provided highlight the widespread importance of this thermodynamic principle in both natural and man-made processes. By grasping the concept of enthalpy change and its relationship to heat transfer, we gain valuable insights into the energetic nature of chemical and physical transformations in our world.
Latest Posts
Latest Posts
-
Why Is Water Necessary For Life
Apr 02, 2025
-
The Process Of Independent Assortment Refers To
Apr 02, 2025
-
Is Table Salt Homogeneous Or Heterogeneous
Apr 02, 2025
-
What Part Of Bacteria Cell Helps It Move
Apr 02, 2025
-
Is The Organic Layer On The Top Or Bottom
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Delta H Is Negative Exothermic Or Endothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
