Density Is A Property Of Matter
Muz Play
Apr 06, 2025 · 6 min read
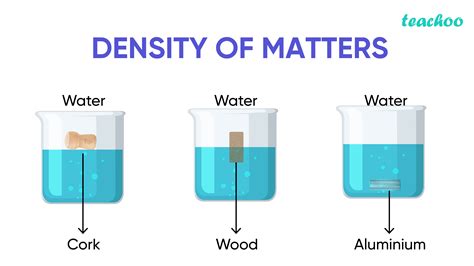
Table of Contents
Density: A Fundamental Property of Matter
Density, a cornerstone concept in physics and chemistry, is a fundamental property of matter that describes how much mass is packed into a given volume. Understanding density is crucial across numerous scientific disciplines, from understanding the behavior of gases in the atmosphere to designing materials for specific applications. This comprehensive guide will explore the intricacies of density, its calculation, applications, and its variations in different states of matter.
Defining Density
Density (ρ, pronounced "rho") is defined as the mass (m) of a substance per unit volume (V). The formula is concise and straightforward:
ρ = m/V
The standard unit for density in the International System of Units (SI) is kilograms per cubic meter (kg/m³). However, other units are commonly used depending on the context, such as grams per cubic centimeter (g/cm³) for solids and liquids, and grams per liter (g/L) or kilograms per cubic meter (kg/m³) for gases. The choice of unit often depends on the scale of the measurement and the material being studied.
Key takeaway: Density is an intensive property, meaning it doesn't depend on the amount of substance present. A teaspoon of water and a swimming pool of water have the same density. This contrasts with extensive properties like mass and volume, which do depend on the amount of substance.
Calculating Density: A Step-by-Step Guide
Calculating the density of a substance involves determining its mass and volume. The process differs slightly depending on whether you're dealing with a regularly shaped object or an irregularly shaped object.
Density Calculation for Regularly Shaped Objects
For objects with regular shapes (cubes, spheres, cylinders, etc.), calculating the volume is straightforward using geometric formulas.
-
Measure the mass: Use a balance or scale to accurately measure the mass of the object in grams or kilograms.
-
Calculate the volume: Use the appropriate geometric formula to determine the volume based on the object's dimensions (length, width, height, radius, etc.). For example:
- Cube: Volume = side³
- Sphere: Volume = (4/3)πr³
- Cylinder: Volume = πr²h
-
Calculate the density: Divide the mass by the volume using the formula ρ = m/V.
Density Calculation for Irregularly Shaped Objects
Determining the volume of irregularly shaped objects requires a slightly different approach, often involving water displacement.
-
Measure the mass: As before, use a balance or scale to measure the mass of the object.
-
Determine the volume using water displacement:
- Fill a graduated cylinder or other suitable container with a known volume of water.
- Carefully submerge the object completely in the water.
- Record the new water level.
- The difference between the initial and final water levels represents the volume of the object.
-
Calculate the density: Divide the mass by the volume as before (ρ = m/V).
Density Variations Across States of Matter
Density is significantly influenced by the state of matter (solid, liquid, or gas).
Density of Solids
Solids generally have the highest densities compared to liquids and gases. This is because the particles in a solid are tightly packed together, resulting in a high mass per unit volume. The density of a solid can vary depending on its crystal structure, temperature, and pressure. For example, the density of ice is slightly lower than the density of liquid water, which is why ice floats on water.
Density of Liquids
Liquids have intermediate densities compared to solids and gases. The particles in a liquid are closer together than in a gas but not as tightly packed as in a solid. The density of a liquid is affected by temperature and pressure, with density generally decreasing as temperature increases and increasing as pressure increases.
Density of Gases
Gases have the lowest densities because their particles are widely spaced and move freely. The density of a gas is highly dependent on temperature and pressure. Higher temperatures lead to increased particle movement and lower density, while higher pressures force particles closer together, increasing density. This relationship is described by the Ideal Gas Law.
Applications of Density
The concept of density finds extensive applications in various fields:
-
Material Science: Density is a crucial factor in material selection for engineering applications. Choosing materials with appropriate densities is critical for structural integrity, weight considerations, and overall performance.
-
Geology: Density measurements help geologists understand the composition and structure of the Earth's layers. Differences in density drive plate tectonics and other geological processes.
-
Oceanography: Density variations in seawater due to temperature and salinity influence ocean currents and marine life distribution.
-
Meteorology: Density differences in air masses drive weather patterns and atmospheric circulation. Changes in air density affect the flight of airplanes and the behavior of weather balloons.
-
Medicine: Density measurements are used in medical imaging techniques like bone density scans and DEXA scans (Dual-energy X-ray absorptiometry) to assess bone health.
-
Archeology: Archaeologists use density measurements to analyze artifacts and determine the composition of ancient materials.
Factors Affecting Density
Several factors can influence the density of a substance:
-
Temperature: Generally, an increase in temperature causes a decrease in density. This is because the particles expand, occupying a larger volume while the mass remains constant. The exception to this is water, which has a maximum density at 4°C.
-
Pressure: An increase in pressure generally leads to an increase in density. Higher pressure forces particles closer together, reducing the volume while the mass remains constant.
-
Composition: The chemical composition of a substance dictates its density. Different elements and molecules have different atomic masses and packing efficiencies, resulting in different densities.
-
Phase: As mentioned earlier, the state of matter significantly influences density. Solids have higher densities than liquids, and liquids have higher densities than gases.
Density and Buoyancy
Density plays a critical role in buoyancy, which describes the ability of an object to float in a fluid (liquid or gas). An object will float if its average density is less than the density of the fluid. Conversely, it will sink if its average density is greater than the density of the fluid. This principle explains why wood floats in water (lower density) and rocks sink (higher density).
Advanced Concepts: Relative Density and Specific Gravity
-
Relative Density: Relative density is the ratio of the density of a substance to the density of a reference substance at a specified temperature and pressure. Water is often used as the reference substance.
-
Specific Gravity: Specific gravity is a dimensionless quantity that is numerically equivalent to relative density. It represents the ratio of the density of a substance to the density of water at 4°C.
Conclusion
Density is a fundamental property of matter with wide-ranging implications across various scientific disciplines and engineering applications. Understanding the concept of density, its calculation, and the factors that influence it is essential for comprehending the behavior of matter in different states and environments. From predicting the behavior of weather systems to designing innovative materials, density remains a critical concept for both scientific research and technological advancements. Further explorations into the fascinating world of density can reveal even deeper insights into the nature of matter and its interactions.
Latest Posts
Latest Posts
-
How To Interpret A Karyotype Answer Key
Apr 09, 2025
-
The Form Acidic Compounds With Hydrogen
Apr 09, 2025
-
What Is The Purpose Of Age Structures
Apr 09, 2025
-
What Is The Difference Between Glycogen And Starch
Apr 09, 2025
-
Melting Ice Physical Or Chemical Change
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Density Is A Property Of Matter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.