Density Of Water 22 Degrees Celsius
Muz Play
Mar 30, 2025 · 5 min read
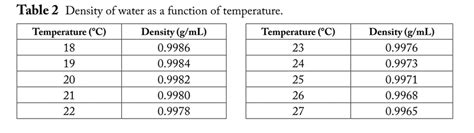
Table of Contents
Density of Water at 22 Degrees Celsius: A Deep Dive
The density of water, a seemingly simple concept, plays a crucial role in numerous scientific fields and everyday life. Understanding its properties, especially at specific temperatures like 22 degrees Celsius, is vital for various applications, from accurate laboratory measurements to environmental modeling. This article delves into the intricacies of water density at 22°C, exploring its value, influencing factors, and practical implications.
What is the Density of Water at 22 Degrees Celsius?
The density of water isn't a constant; it varies with temperature and pressure. At 22 degrees Celsius (approximately room temperature) and standard atmospheric pressure (1 atm), the density of pure water is approximately 997.77 kg/m³. This is often rounded to 998 kg/m³ for practical purposes. It's crucial to note that this value can fluctuate slightly based on the purity of the water and the precision of the measuring instruments. The presence of dissolved salts, minerals, or other impurities can significantly alter the density.
Understanding Density: A Quick Refresher
Before delving deeper, let's briefly recap the concept of density. Density (ρ) is defined as the mass (m) of a substance per unit volume (V):
ρ = m/V
The unit of density is typically kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³). One gram per cubic centimeter is equivalent to one thousand kilograms per cubic meter.
Factors Affecting Water Density
Several factors contribute to the variations in water density, even at a seemingly fixed temperature like 22°C:
1. Temperature: The Dominant Factor
Temperature is the most significant factor influencing water density. As temperature increases, the kinetic energy of water molecules increases, causing them to move further apart. This increased intermolecular spacing leads to a decrease in density. Conversely, as temperature decreases, the molecules move closer together, resulting in higher density. This relationship, however, isn't perfectly linear; water exhibits an anomalous behavior around 4°C.
2. Pressure: A Less Significant Influence at Room Temperature
Pressure also affects water density. Increasing pressure forces the water molecules closer together, thereby increasing density. However, at room temperature and the pressures typically encountered in everyday life and most laboratory settings, the effect of pressure on water density is relatively minor compared to the influence of temperature. The compressibility of water is low.
3. Salinity: The Impact of Dissolved Salts
The presence of dissolved salts and minerals significantly increases the density of water. Seawater, for example, has a higher density than freshwater due to the dissolved salts. This difference in density drives ocean currents and influences marine ecosystems. The higher the salinity, the higher the density, even at the same temperature and pressure.
4. Purity: The Role of Impurities
Any impurities present in the water sample, whether dissolved gases, organic matter, or other substances, will affect its density. Pure water will have a slightly lower density than water containing impurities. The extent of the impact depends on the nature and concentration of the impurities.
The Anomalous Behavior of Water: A Unique Property
Water exhibits a unique property known as its anomalous expansion. Unlike most substances, water's density increases as its temperature decreases until it reaches 4°C. Below 4°C, the density of water starts to decrease as the temperature continues to fall. This means that ice (water at 0°C) is less dense than liquid water at 4°C, causing ice to float. This seemingly simple phenomenon has profound implications for aquatic life and the Earth's climate.
Practical Applications of Water Density at 22°C
The knowledge of water density at 22°C, and its variations, finds applications in diverse fields:
1. Oceanography and Hydrology: Studying Water Bodies
Oceanographers and hydrologists rely on precise density measurements to understand ocean currents, water mixing, and the distribution of various substances within water bodies. Density differences drive many natural processes, including thermohaline circulation, a crucial element of the global climate system.
2. Meteorology: Weather Forecasting and Climate Modeling
Water density is a crucial parameter in meteorological models that predict weather patterns and climate change. The density of water vapor in the atmosphere impacts atmospheric pressure and weather systems. Accurate density measurements are incorporated into sophisticated climate models to predict future climatic scenarios.
3. Chemistry and Biochemistry: Laboratory Measurements
Accurate density measurements are essential in various chemical and biochemical experiments. Determining the concentration of solutions, calculating molarity, and performing other quantitative analyses often depend on knowing the density of the solvent (water in many cases).
4. Engineering: Fluid Dynamics and Design
Engineers use density values in designing systems involving water flow, such as pipes, dams, and hydraulic systems. Understanding density variations due to temperature and pressure is crucial for optimal design and preventing potential issues.
5. Food and Beverage Industry: Quality Control and Processing
The density of water is important in food and beverage processing. Measuring the density of solutions and mixtures helps ensure consistency and quality in products. It's also crucial in applications like determining the sugar content of drinks.
Measuring Water Density: Techniques and Methods
Several methods exist for accurately determining water density:
- Pycnometer: A pycnometer is a specialized glass vessel used to measure the density of liquids. The mass of a known volume of water is measured, allowing calculation of density.
- Hydrometer: A hydrometer is a simple device that floats in the liquid, with a calibrated scale indicating the density. It's a common tool for measuring the density of solutions and liquids.
- Digital Density Meters: Modern digital density meters use advanced technologies, such as oscillating U-tubes, to provide accurate and rapid density measurements. These devices are commonly used in laboratories and industrial settings.
Conclusion: The Significance of Precise Density Knowledge
The density of water at 22°C, while seemingly a simple value, represents a critical parameter with far-reaching implications across various scientific and engineering disciplines. Understanding the factors that influence water density, its variations, and its practical applications are crucial for a wide range of studies and technological advancements. From comprehending the intricacies of ocean currents to designing efficient hydraulic systems, precise knowledge of water density is essential for accurate modeling, analysis, and problem-solving. Further research into the nuances of water density, particularly under different environmental conditions, will continue to improve our understanding of natural processes and enhance technological capabilities.
Latest Posts
Latest Posts
-
Root Mean Square Velocity Of Gas
Apr 01, 2025
-
Where Is The Respiratory Center Located In The Brain
Apr 01, 2025
-
What Is The Difference Between Cellular Respiration And Fermentation
Apr 01, 2025
-
What Are Two Functional Groups Found In Amino Acids
Apr 01, 2025
-
Foundations Of Education 13th Edition Pdf Free Download
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Density Of Water 22 Degrees Celsius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
