Describe The Three Structural Components Of An Rna Nucleotide Monomer.
Muz Play
Mar 16, 2025 · 6 min read
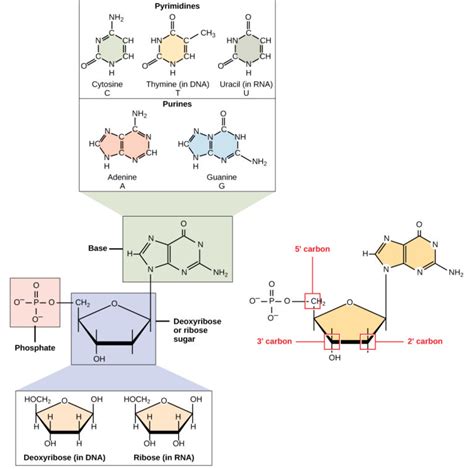
Table of Contents
Decoding the RNA Nucleotide: A Deep Dive into its Three Structural Components
Ribonucleic acid (RNA) is a fundamental molecule of life, playing crucial roles in protein synthesis, gene regulation, and even catalysis. Understanding its structure is key to understanding its function. At its most basic level, RNA, like DNA, is a polymer composed of individual nucleotide monomers. Each of these monomers possesses three essential components: a nitrogenous base, a pentose sugar, and a phosphate group. Let's explore each in detail.
1. The Nitrogenous Base: The Information Carrier
The nitrogenous base is the information-carrying component of the RNA nucleotide. Unlike DNA, which uses the bases adenine (A), guanine (G), cytosine (C), and thymine (T), RNA utilizes uracil (U) instead of thymine. This seemingly small difference has significant implications for RNA's structure and function. Let's examine each base individually:
Adenine (A):
Adenine is a purine base, meaning it possesses a double-ring structure consisting of a six-membered ring fused to a five-membered ring. It forms hydrogen bonds with uracil (U) in RNA, a crucial interaction in RNA secondary structure formation and its interaction with other molecules. Adenine's role extends beyond simple base pairing. It's also a key component of ATP (adenosine triphosphate), the energy currency of the cell. The presence of adenine in RNA nucleotides contributes to its diverse functional roles.
Guanine (G):
Guanine, another purine base, also features a double-ring structure. It forms three hydrogen bonds with cytosine (C) in RNA, a stronger interaction than the adenine-uracil pairing. This stronger bond contributes to the stability of RNA secondary structures, particularly in regions requiring more structural rigidity. The specific positioning of guanine within RNA molecules often dictates their interactions with proteins and other RNA molecules. Guanine's unique chemical properties influence RNA folding and function.
Cytosine (C):
Cytosine is a pyrimidine base, possessing a single six-membered ring structure. It forms three hydrogen bonds with guanine (G), establishing a stable base pair crucial for RNA secondary structure formation. The presence of cytosine influences the overall stability and folding patterns of RNA molecules. Its interaction with guanine contributes to the overall structural integrity of various RNA types, from transfer RNA (tRNA) to ribosomal RNA (rRNA).
Uracil (U):
Uracil, a pyrimidine base, replaces thymine in RNA. It forms two hydrogen bonds with adenine (A), a weaker interaction compared to the guanine-cytosine pairing. This difference in bonding strength contributes to RNA's generally less stable structure compared to DNA. The use of uracil instead of thymine is believed to have evolutionary significance, perhaps related to uracil's greater susceptibility to degradation, which might play a role in RNA's shorter lifespan compared to DNA. The substitution of uracil for thymine is a defining characteristic that distinguishes RNA from DNA.
2. The Pentose Sugar: Ribose – The Backbone's Foundation
The pentose sugar in RNA nucleotides is ribose, a five-carbon sugar. Ribose is a crucial structural component because it provides the backbone to which the nitrogenous base and phosphate group are attached. The presence of a hydroxyl (-OH) group on the 2' carbon of ribose is a key difference between RNA and DNA, which has a hydrogen atom instead. This hydroxyl group is involved in RNA's increased reactivity and susceptibility to hydrolysis, contributing to its relatively shorter lifespan compared to DNA.
The specific arrangement of the ribose molecule determines the orientation of the other components of the nucleotide, influencing the overall three-dimensional structure of the RNA molecule. The hydroxyl group on the 2' carbon is not only responsible for RNA's instability but also plays a role in its catalytic activity, explaining the existence of ribozymes—catalytic RNA molecules. The ribose sugar provides the structural framework for the RNA polymer, offering an environment conducive to the base pairings and secondary structure formations that are critical to RNA's functions.
Ribose vs. Deoxyribose: A Key Distinction
The presence of the 2'-hydroxyl group in ribose is the primary structural difference between RNA and DNA. DNA uses deoxyribose, a sugar lacking this hydroxyl group on the 2' carbon. This seemingly minor difference has significant consequences:
- Hydrolysis: The 2'-hydroxyl group in ribose makes RNA more susceptible to hydrolysis (breakdown by water), contributing to its generally shorter lifespan than DNA.
- Reactivity: The 2'-hydroxyl group increases RNA's reactivity, allowing it to participate in diverse catalytic reactions (e.g., as ribozymes).
- Secondary Structure: The 2'-hydroxyl group influences the conformation of RNA, leading to different secondary structures compared to DNA.
3. The Phosphate Group: Linking the Monomers
The phosphate group is the third crucial component of an RNA nucleotide. It's a negatively charged group (PO43-) which acts as a bridge, linking the 3' carbon of one ribose sugar to the 5' carbon of the next ribose sugar in the RNA chain. This creates the phosphodiester bond, the backbone of the RNA polymer. The negatively charged phosphate groups give RNA its overall negative charge, affecting its interactions with proteins and other molecules.
The phosphate group's location is critical. The phosphodiester bond creates a 5' to 3' polarity in the RNA chain, which is essential for understanding RNA synthesis, processing, and function. The linear sequence of nucleotides, determined by the order of the nitrogenous bases, defines the primary structure of RNA. This primary sequence then folds into complex secondary and tertiary structures crucial for its biological activity.
The Role of Phosphate in RNA Function:
Beyond simply linking nucleotides, the phosphate groups play a more active role in RNA function:
- Charge: The negative charge attracts positively charged ions and proteins, influencing RNA's interactions with its environment.
- Flexibility: The phosphodiester backbone allows for RNA to adopt a range of flexible conformations, important for its diverse roles.
- Enzyme Interactions: The phosphate groups are often involved in interactions with enzymes involved in RNA metabolism and processing.
Conclusion: The Interplay of Structure and Function
The three components—the nitrogenous base, the ribose sugar, and the phosphate group—work in concert to give RNA its unique properties and diverse functionalities. The sequence of nitrogenous bases dictates the genetic information carried by the RNA molecule. The ribose sugar provides the backbone for the polymer, while the phosphate groups link the monomers and contribute to the overall charge and flexibility of the molecule. The interplay between these three components underlies RNA's crucial role in various cellular processes. Understanding the structure of the RNA nucleotide is fundamental to understanding the complexity and diversity of RNA’s functions in life. Further research continues to unravel the subtle nuances of RNA structure and its implications for biological function. This knowledge forms the basis for advancements in various fields, including medicine, biotechnology, and our understanding of life's fundamental processes.
Latest Posts
Latest Posts
-
A Reflex That Causes Muscle Relaxation And Lengthening In Response
Mar 17, 2025
-
Compare And Contrast Magnification And Resolution
Mar 17, 2025
-
Effective Nuclear Charge Vs Nuclear Charge
Mar 17, 2025
-
What Is The Opposite Of Sublimation
Mar 17, 2025
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Describe The Three Structural Components Of An Rna Nucleotide Monomer. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
