Determination Of A Solubility Product Constant
Muz Play
Mar 19, 2025 · 7 min read
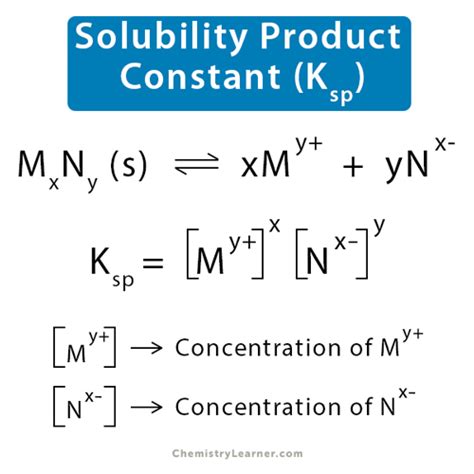
Table of Contents
Determination of a Solubility Product Constant: A Comprehensive Guide
The solubility product constant, Ksp, is a crucial concept in chemistry, quantifying the extent to which a sparingly soluble ionic compound dissolves in an aqueous solution. Understanding and determining this value is essential for various applications, including environmental chemistry, pharmaceutical sciences, and geological studies. This comprehensive guide delves into the theoretical underpinnings of Ksp, explores various methods for its determination, and discusses the factors that influence its value.
Understanding the Solubility Product Constant (Ksp)
The solubility product constant, Ksp, is an equilibrium constant that describes the equilibrium between a solid ionic compound and its constituent ions in a saturated solution. For a general ionic compound, MxAy, which dissolves according to the equation:
MxAy(s) ⇌ xM<sup>y+</sup>(aq) + yA<sup>x-</sup>(aq)
The Ksp expression is given by:
Ksp = [M<sup>y+</sup>]<sup>x</sup>[A<sup>x-</sup>]<sup>y</sup>
Where:
- [M<sup>y+</sup>] represents the molar concentration of the cation M<sup>y+</sup>
- [A<sup>x-</sup>] represents the molar concentration of the anion A<sup>x-</sup>
It's crucial to remember that the solid, MxAy(s), is not included in the Ksp expression because its concentration remains constant in a saturated solution. The Ksp value is temperature-dependent; increasing temperature generally increases solubility and hence the Ksp value.
Significance of Ksp
The Ksp value provides valuable information about the solubility of a compound. A smaller Ksp value indicates a lower solubility, meaning the compound is less likely to dissolve in water. Conversely, a larger Ksp value implies higher solubility. This information is critical in various fields:
- Environmental Chemistry: Predicting the fate of pollutants in water bodies.
- Pharmaceutical Sciences: Formulating drugs with desired solubility characteristics for optimal bioavailability.
- Geochemistry: Understanding mineral formation and dissolution processes in geological systems.
- Analytical Chemistry: Developing methods for separating and analyzing different ions in mixtures.
Methods for Determining Ksp
Several experimental techniques can be employed to determine the Ksp of a sparingly soluble ionic compound. The choice of method depends on the compound's properties and the available instrumentation.
1. Saturation Method
This is the most straightforward method. It involves preparing a saturated solution of the sparingly soluble salt by adding excess solid to a known volume of water. After allowing sufficient time for equilibrium to be established, the solution is filtered to remove any undissolved solid. The concentration of the ions in the saturated solution is then determined using appropriate analytical techniques.
Steps Involved:
- Preparation of Saturated Solution: Add an excess of the solid compound to a known volume of distilled water. Stir the mixture vigorously for an extended period to ensure equilibrium.
- Filtration: Filter the saturated solution to remove any undissolved solid.
- Concentration Determination: Use techniques like titration, spectrophotometry, or atomic absorption spectroscopy to determine the concentration of the dissolved ions. The choice depends on the nature of the ions.
- Ksp Calculation: Substitute the determined ion concentrations into the Ksp expression to calculate the solubility product constant.
Example: If the concentration of M<sup>y+</sup> is found to be 0.001 M and the concentration of A<sup>x-</sup> is 0.001 M for the compound MA, the Ksp would be calculated as:
Ksp = [M<sup>+</sup>][A<sup>-</sup>] = (0.001)(0.001) = 1 x 10<sup>-6</sup>
2. Conductimetric Method
This method exploits the relationship between the conductivity of a solution and the concentration of dissolved ions. A saturated solution of the sparingly soluble salt is prepared, and its conductivity is measured using a conductivity meter. The conductivity is directly proportional to the concentration of ions. By calibrating the meter with solutions of known ionic strength, the ion concentrations in the saturated solution can be determined and used to calculate Ksp.
Advantages:
- Relatively quick and simple.
- Suitable for compounds with moderate to high solubility.
Limitations:
- Accuracy may be affected by the presence of other electrolytes.
3. Potentiometric Method
The potentiometric method utilizes ion-selective electrodes (ISEs) to measure the activity or concentration of specific ions in a saturated solution. An ISE specific to the cation or anion of interest is immersed in the saturated solution, and the potential difference between the ISE and a reference electrode is measured. This potential difference is directly related to the ion concentration.
Advantages:
- High sensitivity and selectivity.
- Suitable for low concentrations.
Limitations:
- Requires specialized equipment.
- Calibration is crucial for accurate results.
4. Spectrophotometric Method
This method is applicable to colored compounds or compounds that form colored complexes with suitable reagents. A saturated solution of the sparingly soluble salt is prepared, and the absorbance of the solution is measured using a spectrophotometer at a specific wavelength. The absorbance is related to the concentration of the dissolved ions through the Beer-Lambert law (A = εbc). By using a calibration curve, the concentration can be determined, and Ksp can be calculated.
Advantages:
- High sensitivity and accuracy.
- Suitable for low concentrations.
Limitations:
- Requires a suitable wavelength for measurement.
- The compound must be colored or form a colored complex.
Factors Affecting Ksp
Several factors influence the value of the solubility product constant:
1. Temperature
As mentioned earlier, temperature significantly impacts solubility. Generally, increasing the temperature increases the solubility of most ionic compounds, leading to a higher Ksp value. This is because increased kinetic energy facilitates the disruption of the ionic lattice and promotes dissolution. However, this is not a universal rule, and some compounds exhibit unusual temperature dependence.
2. Common Ion Effect
The presence of a common ion in the solution significantly reduces the solubility of a sparingly soluble salt. This is because the equilibrium shifts to the left, according to Le Chatelier's principle, minimizing the increase in the concentration of the common ion. For example, adding a soluble chloride salt to a saturated solution of silver chloride (AgCl) will decrease the solubility of AgCl.
3. pH
The pH of the solution can significantly affect the solubility of salts of weak acids or bases. For instance, the solubility of metal hydroxides increases with increasing pH (decreasing H<sup>+</sup> concentration), as the hydroxide ions are consumed in the neutralization reaction.
4. Complex Ion Formation
The formation of complex ions can substantially increase the solubility of a sparingly soluble salt. If a ligand forms a stable complex with the metal cation, it removes the cation from solution, shifting the equilibrium to the right, and increasing the solubility of the salt.
5. Ionic Strength
The presence of other electrolytes in the solution can affect the solubility of a sparingly soluble salt through changes in ionic strength. High ionic strength can decrease the activity coefficients of the ions, potentially increasing their apparent solubility and hence influencing the measured Ksp.
Applications of Ksp
The determination and understanding of Ksp values find broad applications in various scientific and engineering fields:
- Predicting Precipitation: Ksp values are crucial for predicting whether a precipitate will form when two solutions are mixed. If the ion product (Qsp) exceeds the Ksp, a precipitate will form.
- Selective Precipitation: Ksp values allow for the selective precipitation of ions from a mixture. By carefully controlling the concentration of a precipitating agent, one ion can be selectively precipitated while others remain in solution.
- Qualitative Analysis: Ksp values are used in qualitative analysis to identify unknown ions based on their precipitation behavior.
- Solubility Control: In various industrial processes, controlling the solubility of compounds is crucial. Ksp values provide the necessary information to achieve the desired solubility.
Conclusion
Determining the solubility product constant (Ksp) is essential for understanding and predicting the solubility behavior of sparingly soluble ionic compounds. Various methods exist for its determination, each with its advantages and limitations. It's crucial to consider the factors that influence Ksp, including temperature, common ion effect, pH, complex ion formation, and ionic strength. The Ksp value is a powerful tool with wide-ranging applications across numerous scientific disciplines. A thorough understanding of its determination and significance is vital for successful applications in various fields.
Latest Posts
Latest Posts
-
Example Of A Formal Lab Report For Chemistry
Mar 19, 2025
-
The Fluid Filled Area Within The Chloroplast Is Called The
Mar 19, 2025
-
How To Find First Term Of Arithmetic Sequence
Mar 19, 2025
-
Nursing Interventions For Patients With Schizophrenia
Mar 19, 2025
-
Three Important Parts Of Microscope Care
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Determination Of A Solubility Product Constant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
