Difference Between Atomic Mass And Molar Mass
Muz Play
Apr 06, 2025 · 6 min read
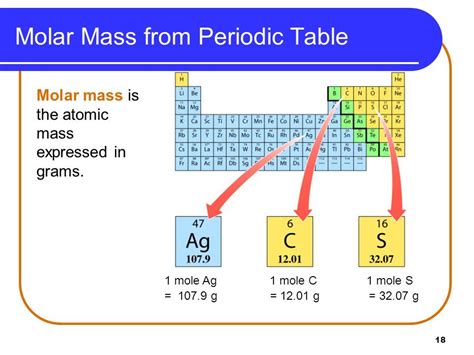
Table of Contents
Atomic Mass vs. Molar Mass: Understanding the Difference
Understanding the fundamental concepts of atomic mass and molar mass is crucial for anyone delving into the world of chemistry. While closely related, these two terms represent different aspects of matter's quantity and weight. This comprehensive guide will delve into the distinctions, exploring their definitions, calculations, applications, and the common pitfalls to avoid when working with them.
What is Atomic Mass?
Atomic mass, also known as atomic weight, refers to the mass of a single atom of a specific element. It's expressed in atomic mass units (amu), where one amu is defined as one-twelfth the mass of a carbon-12 atom. It's important to note that this isn't a simple whole number for most elements because it accounts for the average mass of all naturally occurring isotopes of that element.
Isotopes and Their Role in Atomic Mass
Isotopes are atoms of the same element that possess the same number of protons but differ in the number of neutrons. This difference in neutron count leads to variations in their atomic mass. For instance, carbon has two primary stable isotopes: carbon-12 (⁶¹²C) and carbon-13 (⁶¹³C). Carbon-12 constitutes the majority of naturally occurring carbon, influencing the overall atomic mass.
The atomic mass listed on the periodic table for each element is a weighted average reflecting the abundance of each isotope in nature. This weighted average takes into account the mass of each isotope and its relative abundance. The formula for calculating the average atomic mass is:
Average Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + ...
For example, if an element has two isotopes, one with a mass of 10 amu and 80% abundance, and another with a mass of 12 amu and 20% abundance, the average atomic mass would be:
(10 amu × 0.80) + (12 amu × 0.20) = 10.4 amu
This weighted average is what you'll find on the periodic table, representing the typical atomic mass of an atom of that element.
Determining Atomic Mass
The determination of atomic mass involves sophisticated techniques, primarily mass spectrometry. This instrument separates ions based on their mass-to-charge ratio, allowing scientists to precisely measure the mass of each isotope and its relative abundance. This data is then used to calculate the weighted average atomic mass as previously described.
What is Molar Mass?
Molar mass, on the other hand, represents the mass of one mole of a substance. A mole is a fundamental unit in chemistry, defined as the amount of a substance that contains Avogadro's number (approximately 6.022 × 10²³) of entities (atoms, molecules, ions, etc.). The molar mass is expressed in grams per mole (g/mol).
The Significance of Avogadro's Number
Avogadro's number is a constant that provides a link between the microscopic world of atoms and molecules and the macroscopic world of grams and moles. It allows chemists to work with large quantities of atoms and molecules while still maintaining consistency in their calculations.
Calculating Molar Mass
The molar mass of an element is numerically equal to its atomic mass but expressed in grams per mole. For example, the atomic mass of carbon is approximately 12 amu; therefore, its molar mass is 12 g/mol. This means that one mole of carbon atoms weighs approximately 12 grams.
For compounds, the molar mass is calculated by summing the molar masses of all the atoms present in the chemical formula. For instance, to calculate the molar mass of water (H₂O), we would add the molar mass of two hydrogen atoms (2 × 1.01 g/mol) and one oxygen atom (16.00 g/mol):
2(1.01 g/mol) + 16.00 g/mol = 18.02 g/mol
This indicates that one mole of water molecules weighs approximately 18.02 grams.
Key Differences Between Atomic Mass and Molar Mass
The following table summarizes the key distinctions between atomic mass and molar mass:
| Feature | Atomic Mass | Molar Mass |
|---|---|---|
| Definition | Mass of a single atom | Mass of one mole of a substance |
| Unit | Atomic mass units (amu) | Grams per mole (g/mol) |
| Scale | Microscopic | Macroscopic |
| Value for Elements | Numerically equal to molar mass (but in amu) | Numerically equal to atomic mass (but in g/mol) |
| Calculation | Weighted average of isotopes' masses | Sum of atomic masses of constituent atoms in a compound |
Applications of Atomic Mass and Molar Mass
Both atomic mass and molar mass are essential tools in various chemical calculations and applications:
-
Stoichiometry: Molar mass is fundamental in stoichiometric calculations, enabling the conversion between mass and moles, which is crucial for determining reactant and product quantities in chemical reactions.
-
Empirical and Molecular Formula Determination: Molar mass plays a critical role in determining the empirical and molecular formulas of compounds using experimental data like percentage composition.
-
Concentration Calculations: Molar mass is indispensable for calculating the concentration of solutions expressed in molarity (moles per liter).
-
Gas Laws: The molar mass is important in applying ideal gas law calculations to relate the mass of gas to its volume, pressure and temperature.
-
Nuclear Chemistry: Atomic mass is essential for understanding nuclear reactions and calculations involving isotopes.
-
Spectroscopy: Atomic mass and isotopic abundance data are valuable in various spectroscopic techniques to identify and quantify substances.
Common Mistakes to Avoid
-
Confusing amu and g/mol: Remember that atomic mass is expressed in amu, while molar mass is in g/mol. They are numerically equivalent for elements but represent different concepts.
-
Ignoring Isotopes: Always consider isotopic abundances when calculating the average atomic mass of an element.
-
Incorrect Unit Conversion: Pay close attention to units throughout calculations, ensuring consistent units for mass, moles, and volume.
-
Rounding Errors: Avoid premature rounding in calculations; maintain sufficient significant figures throughout the calculation and round the final answer appropriately.
-
Forgetting Avogadro's Number: Understand the role of Avogadro's number as the link between moles and the number of atoms or molecules.
Conclusion
Atomic mass and molar mass are cornerstones of chemistry. While seemingly similar, understanding their differences is crucial for accurate calculations and a deeper understanding of chemical quantities. By mastering these concepts and avoiding common mistakes, you’ll be well-equipped to tackle a wide range of chemical problems and advance your understanding of the fundamental building blocks of matter. Through consistent practice and attention to detail, you can confidently navigate the world of atomic and molar masses in your chemical endeavors.
Latest Posts
Latest Posts
-
Where Do The Electrons Entering Photosystem Ii Come From
Apr 08, 2025
-
Why Is A Cell A Basic Unit Of Life
Apr 08, 2025
-
How To Calculate Average Molecular Speed
Apr 08, 2025
-
How To Find A Limit On A Graph
Apr 08, 2025
-
What Is The Difference Between Triglycerides And Phospholipids
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Atomic Mass And Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.