Difference Between Column And Thin Layer Chromatography
Muz Play
Mar 17, 2025 · 5 min read
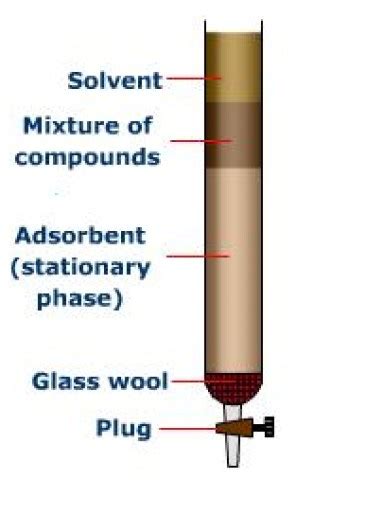
Table of Contents
Delving Deep: Column Chromatography vs. Thin Layer Chromatography
Chromatography, a cornerstone technique in analytical chemistry and biochemistry, boasts a diverse array of methods designed to separate complex mixtures into their individual components. Among these, column chromatography and thin-layer chromatography (TLC) stand out as foundational techniques, both utilizing the principle of differential adsorption to achieve separation. While sharing this fundamental principle, they differ significantly in their methodology, applications, and overall scale of operation. This in-depth article will meticulously dissect the key differences between column chromatography and TLC, exploring their individual strengths, weaknesses, and respective niches within the scientific landscape.
Fundamental Principles: A Shared Foundation
Both column chromatography and TLC rely on the principle of differential adsorption. This involves a stationary phase (a solid material) and a mobile phase (a liquid or gas). The mixture to be separated is introduced into the system, and its components interact differently with both phases. Components with a stronger affinity for the stationary phase will move more slowly, while those with a stronger affinity for the mobile phase will move more quickly. This differential migration leads to the separation of the components.
The key difference lies in the physical manifestation of the stationary and mobile phases. In column chromatography, the stationary phase is packed into a vertical column, while the mobile phase is a solvent that percolates through the column, carrying the mixture components with it. In thin-layer chromatography, the stationary phase is a thin layer of adsorbent material coated onto a solid support (typically a glass or plastic plate), and the mobile phase is a solvent that ascends the plate via capillary action.
Column Chromatography: A Comprehensive Overview
Column chromatography, a workhorse technique in preparative chemistry, excels in separating larger quantities of mixtures. Its ability to handle larger sample sizes makes it particularly valuable for isolating and purifying compounds for further analysis or synthesis.
Types of Column Chromatography:
Column chromatography encompasses various subtypes, each tailored to specific separation challenges:
-
Open Column Chromatography: This classical approach utilizes a glass column packed with the stationary phase. It's simple and cost-effective but can be time-consuming, especially for complex mixtures.
-
Flash Column Chromatography (FCC): This improved version employs positive pressure to accelerate the elution process, significantly reducing separation time. It's widely used in organic chemistry laboratories due to its efficiency and speed.
-
High-Performance Liquid Chromatography (HPLC): This sophisticated technique utilizes highly efficient columns and precise control over the mobile phase to achieve superior separations of complex mixtures, even at trace levels. HPLC is instrumental in analytical chemistry and requires specialized equipment.
Advantages of Column Chromatography:
- High sample capacity: Able to process larger quantities of material.
- Excellent resolution: Can achieve high-quality separations, especially with optimized conditions.
- Scalability: Easily adaptable for various scales, from small analytical separations to large-scale purifications.
- Versatile stationary phases: Offers a wide selection of stationary phases to optimize separations based on polarity, size, or other properties.
Disadvantages of Column Chromatography:
- Time-consuming: Open column chromatography can be quite lengthy.
- Requires specialized equipment: HPLC necessitates advanced instrumentation.
- Solvent consumption: Can require substantial solvent volumes, especially in open column chromatography.
- Less suitable for volatile compounds: Open columns might be less suitable due to evaporation.
Thin Layer Chromatography: A Rapid Screening Technique
Thin layer chromatography (TLC) serves primarily as a rapid and qualitative analytical technique. It's invaluable for monitoring reaction progress, identifying compounds, and assessing the purity of samples. TLC's simplicity and speed make it an indispensable tool in many chemical laboratories.
TLC Methodology:
TLC involves spotting a small amount of the sample mixture onto a TLC plate, then placing the plate in a developing chamber containing the mobile phase. The solvent ascends the plate by capillary action, separating the components based on their differential affinity for the stationary and mobile phases. The separated components appear as spots, enabling identification and analysis.
Advantages of Thin Layer Chromatography:
- Speed and simplicity: Provides rapid results with minimal setup.
- Low cost and ease of use: Requires relatively inexpensive materials and is easily performed.
- Versatility: Compatible with a wide range of solvents and stationary phases.
- Suitable for both qualitative and quantitative analysis: Though primarily qualitative, quantitative analysis is achievable through densitometry.
Disadvantages of Thin Layer Chromatography:
- Limited sample capacity: Can only handle small amounts of sample.
- Lower resolution compared to column chromatography: May not completely separate closely related compounds.
- Semi-quantitative: Provides qualitative information primarily, quantitative data requires further analysis.
- Susceptible to errors: Technique is sensitive to errors in spotting, development, and visualization.
A Comparative Table: Column Chromatography vs. Thin Layer Chromatography
| Feature | Column Chromatography | Thin Layer Chromatography |
|---|---|---|
| Scale | Preparative (large-scale) | Analytical (small-scale) |
| Sample Size | Large | Small |
| Speed | Relatively slow (open column); fast (HPLC) | Very fast |
| Resolution | High (especially HPLC) | Moderate |
| Cost | Can be expensive (HPLC) | Low |
| Complexity | Moderate to high (HPLC) | Low |
| Applications | Purification, isolation, preparative work | Monitoring reactions, identification, purity assessment |
| Quantitative Analysis | Possible with advanced techniques | Possible with densitometry |
Choosing the Right Technique: A Practical Guide
The choice between column chromatography and TLC depends heavily on the specific application and experimental goals.
Choose column chromatography if:
- You need to purify or isolate significant quantities of a compound.
- High resolution separation is crucial.
- You have access to appropriate equipment (for HPLC).
Choose thin-layer chromatography if:
- You need a quick and inexpensive method for identifying compounds or assessing purity.
- You need to monitor a reaction's progress rapidly.
- Only a small sample size is available.
Advanced Considerations and Future Trends
Both column chromatography and TLC are constantly evolving. Advances in stationary phase materials, mobile phase optimization, and detection techniques continually enhance their capabilities. For instance, the development of novel stationary phases with improved selectivity and efficiency continues to expand the applications of both methods. Similarly, advancements in detection methods, such as mass spectrometry (MS) coupled with both techniques, provide more detailed information about the separated components.
Conclusion:
Column chromatography and thin-layer chromatography are indispensable tools in analytical and preparative chemistry. While both rely on the same basic principles, their applications, scales, and complexities differ substantially. Understanding these differences is crucial for selecting the most appropriate technique for a given task, maximizing efficiency, and obtaining reliable results. By carefully considering the advantages and disadvantages of each method, researchers can effectively leverage these powerful techniques to solve diverse analytical and preparative challenges.
Latest Posts
Latest Posts
-
How Catalyst Increases The Rate Of Reaction
May 10, 2025
-
What Is The Measurement Of Pressure
May 10, 2025
-
Biological Polymers Are Produced By The Process Of
May 10, 2025
-
Compare And Contrast K Selected Species And R Selected Species
May 10, 2025
-
What Helps To Distinguish Science From Other Ways Of Knowing
May 10, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Column And Thin Layer Chromatography . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.