Difference Between Solution Suspension And Colloid
Muz Play
Apr 01, 2025 · 6 min read
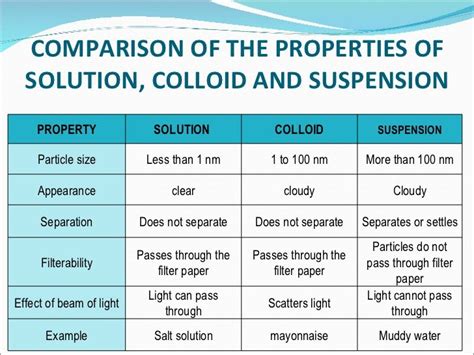
Table of Contents
The Differences Between Solutions, Suspensions, and Colloids: A Deep Dive
Understanding the differences between solutions, suspensions, and colloids is crucial in various scientific fields, from chemistry and biology to materials science and engineering. These three terms describe different types of mixtures, distinguished primarily by the size of the particles involved and how those particles interact with the dispersing medium (usually a liquid). While they might appear similar at first glance, their properties and behaviors differ significantly. This article will explore these differences in detail, offering a comprehensive overview for a wide range of readers.
What is a Solution?
A solution is a homogeneous mixture where one substance, the solute, is uniformly dispersed within another substance, the solvent. The solute particles are incredibly small—typically individual atoms, ions, or small molecules—making them invisible to the naked eye and undetectable by light scattering techniques. They are completely dissolved within the solvent, forming a single phase.
Key Characteristics of Solutions:
- Particle Size: Less than 1 nanometer (nm).
- Homogeneity: Uniform composition throughout; the solute and solvent are indistinguishable.
- Filtration: The solute cannot be separated from the solvent by filtration.
- Sedimentation: The solute particles do not settle out over time.
- Transparency: Solutions are typically transparent. Light passes through without significant scattering.
- Examples: Saltwater (salt dissolved in water), sugar dissolved in water, air (gases dissolved in other gases).
What is a Suspension?
A suspension is a heterogeneous mixture containing larger particles of solute dispersed within a solvent. These particles are visible to the naked eye and readily settle out of the solution if left undisturbed. Unlike solutions, suspensions consist of two distinct phases: the dispersed phase (solute particles) and the continuous phase (solvent).
Key Characteristics of Suspensions:
- Particle Size: Greater than 1000 nanometers (1 micrometer or 1 μm).
- Heterogeneity: Non-uniform composition; the solute particles are visibly distinct from the solvent.
- Filtration: The solute particles can be separated from the solvent by simple filtration.
- Sedimentation: The solute particles settle out of the suspension over time due to gravity.
- Opacity: Suspensions are usually opaque or cloudy due to light scattering by the large particles.
- Examples: Sand in water, mud in water, chalk powder in water.
What is a Colloid?
A colloid falls between a solution and a suspension. It’s a heterogeneous mixture containing particles larger than those in a solution but smaller than those in a suspension. These particles, known as colloidal particles, are typically between 1 and 1000 nanometers in size. They are too small to be seen with the naked eye but large enough to scatter light, resulting in a characteristic Tyndall effect.
Key Characteristics of Colloids:
- Particle Size: Between 1 and 1000 nanometers (1 nm to 1 μm).
- Heterogeneity: Though appearing homogenous to the naked eye, colloids are heterogeneous at the microscopic level.
- Filtration: Colloidal particles are too small to be removed by ordinary filtration but can be separated using techniques like ultrafiltration or centrifugation.
- Sedimentation: Colloidal particles do not settle out under gravity, remaining dispersed due to Brownian motion (random movement caused by collisions with solvent molecules).
- Tyndall Effect: Colloids exhibit the Tyndall effect—the scattering of light by the colloidal particles, making a beam of light visible when passed through the colloid.
- Examples: Milk (fat droplets in water), fog (water droplets in air), blood (cells and proteins in plasma), paint, ink.
Comparing Solutions, Suspensions, and Colloids: A Table Summary
To further clarify the distinctions, the following table summarizes the key differences:
| Feature | Solution | Colloid | Suspension |
|---|---|---|---|
| Particle Size | < 1 nm | 1 nm - 1000 nm | > 1000 nm |
| Homogeneity | Homogeneous | Heterogeneous | Heterogeneous |
| Filtration | Cannot be filtered | Cannot be filtered (ordinary) | Can be filtered |
| Sedimentation | Does not settle | Does not settle (gravity) | Settles readily |
| Appearance | Transparent | Translucent or opaque | Opaque |
| Tyndall Effect | Absent | Present | Absent |
The Tyndall Effect: A Distinguishing Feature of Colloids
The Tyndall effect is a crucial characteristic that differentiates colloids from solutions and suspensions. When a beam of light is passed through a solution, it passes through unobstructed. In a suspension, the light might be partially blocked by the larger particles. However, in a colloid, the light is scattered by the colloidal particles, making the beam of light visible. This scattering effect is due to the interaction of light with the particles, which are large enough to diffract and scatter the light waves.
Applications of Solutions, Suspensions, and Colloids
Solutions, suspensions, and colloids have diverse applications across various industries.
Solutions:
- Medicine: Oral medications often involve dissolving active ingredients in water or other solvents for easy ingestion and absorption.
- Industry: Many chemical reactions and processes require the use of solutions to achieve the desired outcome.
- Everyday Life: The preparation of beverages like tea or coffee involves creating solutions.
Suspensions:
- Medicine: Some medications, like certain antibiotics, are formulated as suspensions to ensure better stability and controlled release of the active ingredient.
- Cosmetics: Many creams and lotions utilize suspensions to provide a uniform delivery of active components.
- Agriculture: Pesticides and herbicides are often delivered as suspensions.
Colloids:
- Food Science: Many food products, including milk, yogurt, and ice cream, are colloids.
- Materials Science: Colloidal materials like nanoparticles are used in various applications, from electronics to medicine.
- Environmental Science: Understanding colloids is essential in studying the behavior of pollutants in water and air.
Factors Affecting the Stability of Colloids
The stability of colloids is critical for many applications. Several factors can influence it:
- Particle Size and Shape: Smaller, uniformly shaped particles tend to form more stable colloids.
- Surface Charge: Particles with similar surface charges repel each other, preventing aggregation and sedimentation.
- Presence of Stabilizing Agents: Substances like emulsifiers and protective colloids can prevent coagulation and maintain the colloidal dispersion.
- Temperature: Changes in temperature can affect the interactions between particles and the solvent, potentially destabilizing the colloid.
Conclusion
The distinction between solutions, suspensions, and colloids lies primarily in the size of the dispersed particles and their consequent behavior. Solutions contain dissolved particles too small to be seen or filtered, suspensions contain large, readily-settling particles, and colloids occupy the intermediate range, exhibiting the Tyndall effect and remaining dispersed due to Brownian motion. Understanding these differences is crucial across numerous scientific and technological fields, enabling advancements in materials science, medicine, food technology, and more. The unique properties of each type of mixture dictate their diverse applications and impact our everyday lives in numerous ways. Further research into the complex behavior of these mixtures continues to yield exciting discoveries and innovations.
Latest Posts
Latest Posts
-
When Does Segregation Occur In Meiosis
Apr 02, 2025
-
A Worm Is Living Inside A Cow
Apr 02, 2025
-
What Plane Divides The Body Into Front And Back Portions
Apr 02, 2025
-
Who Wrote The Opera The Magic Flute
Apr 02, 2025
-
Properties Of Logarithms Worksheet With Answers
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Solution Suspension And Colloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
