Difference Between Tlc And Column Chromatography
Muz Play
Mar 15, 2025 · 6 min read
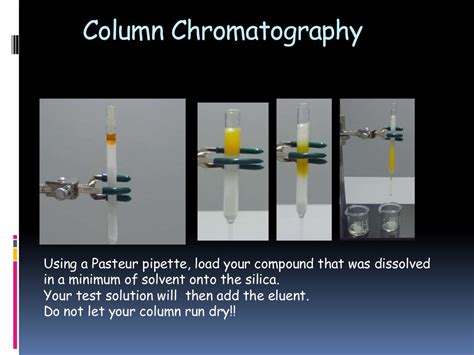
Table of Contents
TLC vs. Column Chromatography: A Comprehensive Guide
Chromatography, a cornerstone technique in chemistry and biochemistry, encompasses various methods for separating mixtures into their individual components. Thin-layer chromatography (TLC) and column chromatography are two widely used techniques, each with its own strengths and limitations. Understanding the key differences between these methods is crucial for selecting the appropriate technique for a specific application. This comprehensive guide delves deep into the distinctions between TLC and column chromatography, covering their principles, procedures, applications, advantages, and disadvantages.
Understanding the Fundamentals: Chromatography Principles
Before diving into the specifics of TLC and column chromatography, let's establish a common ground by understanding the underlying principles of chromatography. Chromatography relies on the differential distribution of compounds between two phases: a stationary phase and a mobile phase.
The stationary phase is a solid or liquid that is fixed in place, typically within a column (in column chromatography) or on a plate (in TLC). The mobile phase is a liquid or gas that moves over the stationary phase, carrying the components of the mixture with it.
The separation of components is achieved based on their differing affinities for the stationary and mobile phases. Compounds with a higher affinity for the stationary phase will move more slowly, while those with a higher affinity for the mobile phase will move faster. This differential migration results in the separation of the mixture's components.
Thin-Layer Chromatography (TLC): A Quick and Simple Technique
TLC is a simple, rapid, and inexpensive chromatographic technique widely used for qualitative analysis. It's frequently employed for monitoring reactions, identifying compounds, and assessing the purity of samples.
TLC: The Procedure
-
Preparation of the TLC Plate: A thin layer of adsorbent material (typically silica gel or alumina) is coated on a glass or plastic plate.
-
Sample Application: A small amount of the sample solution is carefully spotted onto the plate near the bottom edge, using a capillary tube or micropipette.
-
Development: The plate is placed in a developing chamber containing a suitable solvent system (the mobile phase). The solvent ascends the plate by capillary action, carrying the sample components with it.
-
Visualization: Once the solvent front reaches a predetermined height, the plate is removed and allowed to dry. The separated components, often invisible to the naked eye, are visualized using various techniques, such as UV light, iodine staining, or specific chemical reagents.
-
Analysis: The separated components are identified by comparing their Rf (retention factor) values, which represent the distance traveled by the component relative to the distance traveled by the solvent front.
TLC: Advantages and Disadvantages
Advantages:
- Simplicity and Speed: TLC is a relatively quick and easy technique to perform, requiring minimal equipment.
- Low Cost: The materials and equipment needed for TLC are inexpensive.
- Small Sample Size: Only a small amount of sample is required.
- Versatility: A wide range of solvent systems can be used to optimize separation.
Disadvantages:
- Qualitative Primarily: TLC is primarily a qualitative technique, providing limited quantitative information.
- Limited Resolution: TLC offers lower resolution compared to column chromatography, making it less suitable for separating complex mixtures.
- Subjective Analysis: Visual analysis of the spots can be subjective and prone to error.
- Semi-Quantitative at best: While you can sometimes estimate relative amounts based on spot intensity, accurate quantitation is difficult.
Column Chromatography: A Powerful Separation Technique
Column chromatography is a more powerful and versatile technique than TLC, used for both preparative and analytical purposes. It offers higher resolution and allows for the isolation and purification of individual components from complex mixtures.
Column Chromatography: The Procedure
-
Column Packing: A glass column is packed with an adsorbent material (usually silica gel or alumina), creating a uniform bed.
-
Sample Application: The sample is dissolved in a small volume of solvent and carefully applied to the top of the column.
-
Elution: A suitable solvent system (eluting solvent) is then passed through the column, carrying the sample components with it. The elution process can be optimized by using gradient elution, where the solvent composition is gradually changed to improve separation.
-
Fraction Collection: The eluent is collected in fractions, which are then analyzed to identify the separated components. This analysis might involve techniques like TLC, UV-Vis spectroscopy, or other methods.
-
Component Isolation: Once the fractions containing the desired components are identified, they can be further processed to isolate and purify the components. This often involves evaporation of the solvent.
Column Chromatography: Advantages and Disadvantages
Advantages:
- High Resolution: Column chromatography offers significantly higher resolution than TLC, enabling the separation of complex mixtures with closely related components.
- Preparative Scale: It can be scaled up to purify larger quantities of material.
- Quantitative Analysis: With proper instrumentation (e.g., detectors linked to the column), quantitative data can be obtained about the amount of each component.
- Versatile: Different stationary and mobile phases can be used to optimize separations for a wide variety of compounds.
Disadvantages:
- Time-Consuming: Column chromatography is a more time-consuming procedure compared to TLC.
- Higher Cost: It requires more specialized equipment and materials, making it more expensive than TLC.
- Larger Sample Size Required: Often requires larger sample amounts compared to TLC.
- Technical Expertise: Successful column chromatography requires a greater level of technical skill and expertise compared to TLC.
Comparing TLC and Column Chromatography: A Head-to-Head Analysis
| Feature | TLC | Column Chromatography |
|---|---|---|
| Purpose | Primarily qualitative, small-scale | Qualitative and quantitative, preparative-scale |
| Resolution | Low | High |
| Speed | Fast | Slow |
| Cost | Low | High |
| Sample Size | Small | Can range from small to large |
| Complexity | Simple | More complex |
| Quantitation | Difficult, semi-quantitative at best | Readily achievable with appropriate detectors |
| Applications | Reaction monitoring, purity checks | Purification, isolation, and quantitative analysis |
Choosing the Right Technique: TLC or Column Chromatography?
The choice between TLC and column chromatography depends on several factors, including:
- The complexity of the mixture: For simple mixtures, TLC might suffice. For complex mixtures, column chromatography is necessary.
- The quantity of material to be separated: TLC is suitable for small samples, while column chromatography is used for larger quantities.
- The required resolution: If high resolution is needed, column chromatography is the preferred method.
- The need for quantitative analysis: Column chromatography allows for quantitative analysis, while TLC is mainly qualitative.
- Available resources and expertise: The choice may also depend on the available equipment, budget, and technical expertise.
Advanced Techniques and Considerations
Both TLC and column chromatography have evolved, incorporating advanced techniques to improve their efficiency and capabilities. For instance, High-Performance Liquid Chromatography (HPLC) is a highly sophisticated form of column chromatography that utilizes high pressure to achieve superior resolution and speed. Similarly, advancements in detection methods have improved the sensitivity and accuracy of both TLC and column chromatography.
Furthermore, the selection of the appropriate stationary and mobile phases is crucial for optimal separation. The choice depends on the properties of the compounds being separated, and experimentation is often needed to find the best combination. Factors such as polarity, adsorption capacity, and selectivity need careful consideration.
Conclusion
TLC and column chromatography are indispensable techniques in chemistry and related fields. While TLC provides a rapid and simple means of qualitative analysis, column chromatography offers superior resolution and the ability to purify significant quantities of material. Understanding the strengths and weaknesses of each technique, as well as their underlying principles, empowers researchers to select the most appropriate method for a given application and achieve optimal results in their separations. Careful consideration of sample complexity, desired resolution, and available resources is crucial in making the right choice for successful chromatographic analysis.
Latest Posts
Latest Posts
-
A Catalyst Increases The Rate Of A Reaction By
May 09, 2025
-
On What Basis Are Sedimentary Rocks Classified
May 09, 2025
-
Classify Each Description By The Phase Change It Depicts
May 09, 2025
-
Is Gasoline Evaporated A Chemical Change
May 09, 2025
-
How Does Myosin And Actin Interact With Each Other
May 09, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Tlc And Column Chromatography . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.