Diisopropyl Ether Reacts With Concentrated Aqueous Hi
Muz Play
Mar 31, 2025 · 5 min read
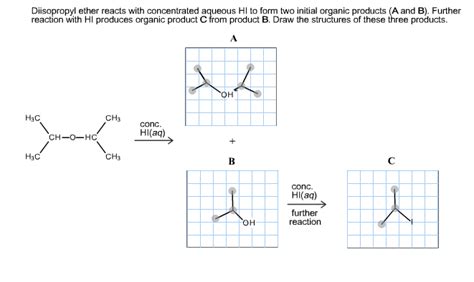
Table of Contents
Diisopropyl Ether and Concentrated Aqueous HI: A Deep Dive into the Reaction Mechanism and Applications
Diisopropyl ether (DIPE), a common solvent, undergoes a fascinating reaction with concentrated aqueous hydroiodic acid (HI). This reaction, seemingly simple on the surface, reveals a rich tapestry of organic chemistry principles, including nucleophilic substitution, carbocation rearrangements, and the influence of reaction conditions. This comprehensive article delves into the intricacies of this reaction, exploring its mechanism, the factors influencing its outcome, and its potential applications.
Understanding the Reactants
Before delving into the reaction itself, let's briefly examine the properties of the reactants: diisopropyl ether and concentrated aqueous HI.
Diisopropyl Ether (DIPE): Structure and Properties
Diisopropyl ether, also known as diisopropyl oxide, is a colorless, volatile liquid with a characteristic ethereal odor. Its structure consists of two isopropyl groups linked by an oxygen atom: (CH<sub>3</sub>)<sub>2</sub>CH-O-CH(CH<sub>3</sub>)<sub>2</sub>. This symmetrical structure is crucial in understanding the reaction with HI. The relatively weak C-O bond is susceptible to cleavage, a key step in the reaction mechanism. Its volatility and solubility properties make it useful as a solvent in various organic reactions, but it's also flammable and should be handled with care.
Concentrated Aqueous HI: A Powerful Nucleophile and Acid
Concentrated hydroiodic acid is a strong acid and a potent nucleophile. The iodide ion (I⁻) is a large, polarizable anion, making it an excellent nucleophile in SN2 reactions. The high concentration of HI ensures a sufficient supply of both H⁺ and I⁻ ions, crucial for the reaction's progression. The acidic nature of HI also plays a significant role, protonating the ether oxygen, activating it towards nucleophilic attack.
The Reaction Mechanism: A Step-by-Step Analysis
The reaction between diisopropyl ether and concentrated aqueous HI proceeds through a series of steps, involving both acid-catalyzed cleavage and nucleophilic substitution. The exact outcome, however, can be influenced by reaction conditions such as temperature and concentration of HI.
Step 1: Protonation of the Ether Oxygen
The reaction initiates with the protonation of the ether oxygen by HI. The oxygen atom, possessing lone pairs of electrons, readily accepts a proton from the acidic HI, forming a protonated ether. This protonation significantly increases the electrophilicity of the carbon atoms adjacent to the oxygen.
(CH<sub>3</sub>)<sub>2</sub>CH-O-CH(CH<sub>3</sub>)<sub>2</sub> + H⁺ → (CH<sub>3</sub>)<sub>2</sub>CH-O⁺H-CH(CH<sub>3</sub>)<sub>2</sub>
Step 2: Nucleophilic Attack by Iodide
The protonated ether is now susceptible to nucleophilic attack by the iodide ion (I⁻). The iodide ion, being a strong nucleophile, attacks one of the carbon atoms bonded to the oxygen. This leads to the cleavage of the C-O bond and the formation of an isopropyl iodide and a protonated isopropyl alcohol.
(CH<sub>3</sub>)<sub>2</sub>CH-O⁺H-CH(CH<sub>3</sub>)<sub>2</sub> + I⁻ → (CH<sub>3</sub>)<sub>2</sub>CHI + (CH<sub>3</sub>)<sub>2</sub>CHOH₂⁺
Step 3: Deprotonation and Further Reaction
The protonated isopropyl alcohol is quickly deprotonated by another iodide ion or a water molecule, yielding isopropyl alcohol. However, the reaction doesn't stop here. Concentrated HI is present in excess, and the isopropyl alcohol undergoes further reaction.
(CH<sub>3</sub>)<sub>2</sub>CHOH₂⁺ + I⁻ → (CH<sub>3</sub>)<sub>2</sub>CHOH + HI
Step 4: Second Nucleophilic Attack and Formation of Second Isopropyl Iodide
The newly formed isopropyl alcohol reacts with HI in a similar manner to the ether. The alcohol's hydroxyl group is protonated, making it a better leaving group, and another nucleophilic attack by an iodide ion leads to the formation of another molecule of isopropyl iodide.
(CH<sub>3</sub>)<sub>2</sub>CHOH + HI → (CH<sub>3</sub>)<sub>2</sub>CHOH₂⁺ + I⁻ → (CH<sub>3</sub>)<sub>2</sub>CHI + H₂O
Overall Reaction and Product Formation
The overall reaction can be summarized as follows:
(CH<sub>3</sub>)<sub>2</sub>CH-O-CH(CH<sub>3</sub>)<sub>2</sub> + 2HI → 2(CH<sub>3</sub>)<sub>2</sub>CHI + H₂O
The major product of the reaction is isopropyl iodide. The reaction is essentially a complete cleavage of the ether linkage, converting the diisopropyl ether into two molecules of isopropyl iodide and water.
Influence of Reaction Conditions
The reaction's efficiency and outcome are significantly affected by reaction conditions:
-
Concentration of HI: Higher concentrations of HI accelerate the reaction by providing a greater concentration of both protons and iodide ions for the nucleophilic attack.
-
Temperature: Higher temperatures generally increase the reaction rate, but excessive heat can lead to side reactions or decomposition of the products.
-
Reaction Time: Sufficient time is required to allow the reaction to proceed to completion.
Potential Applications and Significance
While this specific reaction may not be widely used as a standalone synthetic method, the principles demonstrated have broader implications in organic synthesis:
-
Ether Cleavage: This reaction highlights the susceptibility of ethers, especially those with readily available carbocation intermediates, to acidic cleavage. This knowledge is valuable when working with ether solvents or protecting groups in various synthetic pathways.
-
Nucleophilic Substitution: The reaction showcases the power of iodide as a nucleophile in SN1 and SN2 reactions. Understanding these mechanisms is fundamental in planning many organic transformations.
-
Acid-Catalyzed Reactions: The reaction demonstrates the catalytic role of strong acids in activating functional groups towards nucleophilic attack. This is a prevalent theme in many organic reaction mechanisms.
-
Synthesis of Alkyl Iodides: While isopropyl iodide may not have widespread individual applications, alkyl iodides are important intermediates in organic synthesis, used in numerous coupling reactions and other transformations. This reaction provides a method for their preparation.
Conclusion
The reaction between diisopropyl ether and concentrated aqueous HI is a rich example of acid-catalyzed ether cleavage and nucleophilic substitution. Understanding this reaction, its mechanism, and the influence of reaction conditions offers valuable insights into fundamental organic chemistry principles. While the specific application of this reaction may be limited, the underlying concepts are widely applicable in the design and understanding of numerous organic transformations, reinforcing the importance of exploring seemingly simple reactions for a deeper grasp of organic chemistry. Further research into reaction optimization and potential applications of the resulting alkyl iodides could unveil new possibilities in synthetic organic chemistry. The reaction serves as an excellent teaching tool, highlighting the interplay of acid-base chemistry and nucleophilic substitution reactions.
Latest Posts
Latest Posts
-
What Does Salt Do In Dna Extraction
Apr 02, 2025
-
Why Is Water Necessary For Life
Apr 02, 2025
-
The Process Of Independent Assortment Refers To
Apr 02, 2025
-
Is Table Salt Homogeneous Or Heterogeneous
Apr 02, 2025
-
What Part Of Bacteria Cell Helps It Move
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Diisopropyl Ether Reacts With Concentrated Aqueous Hi . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
