Do Noble Gases Have Ionization Energy
Muz Play
Mar 22, 2025 · 5 min read
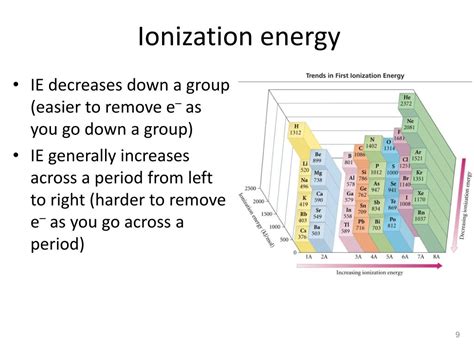
Table of Contents
Do Noble Gases Have Ionization Energy? Understanding the Trends and Exceptions
Noble gases, also known as inert gases, are renowned for their exceptional stability and minimal reactivity. This inherent stability arises from their complete valence electron shells, a feature that significantly impacts their ionization energies. While their high ionization energies are a hallmark of their chemical behavior, it's crucial to understand the nuances and exceptions within this group. This article delves deep into the ionization energy of noble gases, exploring the trends, explaining the underlying principles, and discussing any deviations from the expected patterns.
What is Ionization Energy?
Before we delve into the ionization energy of noble gases, let's establish a clear understanding of the concept itself. Ionization energy (IE) is the minimum energy required to remove an electron from a neutral gaseous atom or ion in its ground electronic state. It's a measure of an atom's ability to hold onto its electrons. The higher the ionization energy, the more difficult it is to remove an electron, indicating stronger electron-nucleus attraction. This energy is typically expressed in kilojoules per mole (kJ/mol) or electronvolts (eV).
Successive Ionization Energies:
It's important to note that ionization energy isn't a single value for an atom. Atoms can undergo successive ionization whereby further electrons are removed after the initial ionization. Each successive ionization energy (IE<sub>1</sub>, IE<sub>2</sub>, IE<sub>3</sub>, etc.) is progressively higher than the previous one. This is because removing an electron alters the electron-to-proton ratio, increasing the effective nuclear charge experienced by the remaining electrons, making them harder to remove.
Ionization Energy of Noble Gases: The General Trend
Noble gases (Helium, Neon, Argon, Krypton, Xenon, and Radon) occupy Group 18 of the periodic table. Their electron configurations are characterized by completely filled valence electron shells (ns²np⁶, except for Helium, which has a filled 1s² shell). This full valence shell is the primary reason for their exceptionally high ionization energies. The general trend within the noble gases is that ionization energy increases down the group.
This trend is directly linked to the atomic radius. As you move down the group, the atomic radius increases. This increase in size leads to a greater distance between the nucleus and the outermost electrons. The increased distance weakens the electrostatic attraction between the nucleus and valence electrons, requiring less energy to remove them. Therefore, ionization energy decreases down the group.
Helium: The Exceptional Case
Helium, the lightest noble gas, presents a unique case. Despite its small size and strong nuclear charge, its ionization energy is lower than that of Neon. Although this seems counterintuitive given the general trend of increasing ionization energy across a period, there is an explanation for this. Helium has a simple electron configuration, 1s², and the two electrons occupy a spherically symmetric orbital. Thus, they experience a significant amount of shielding from one another, decreasing the effective nuclear charge felt by the electrons.
Factors Affecting Ionization Energy of Noble Gases:
Several factors interplay to determine the ionization energy of noble gases:
- Nuclear Charge: A higher nuclear charge leads to a stronger attraction between the nucleus and electrons, increasing the ionization energy.
- Shielding Effect: Inner electrons shield the valence electrons from the full positive charge of the nucleus, reducing the effective nuclear charge and decreasing the ionization energy. The shielding effect is more pronounced in heavier noble gases due to the greater number of inner electrons.
- Atomic Radius: As mentioned earlier, a larger atomic radius leads to weaker electrostatic attraction, hence lower ionization energy.
- Electron-Electron Repulsion: The repulsion between electrons in the same shell can slightly reduce the effective nuclear charge and thus the ionization energy.
Exceptions and Anomalies:
While the general trend of decreasing ionization energy down the noble gas group holds true, subtle variations exist. These deviations are primarily attributed to the complex interplay of factors like electron shielding and inter-electronic repulsion. Precise measurements reveal slight irregularities, but the overall trend remains consistent.
Applications and Significance:
The high ionization energies of noble gases have significant implications in various fields:
- Lighting: Noble gases are used in lighting applications due to their ability to emit light when energized. The energy required to ionize them contributes to the efficiency and properties of these light sources.
- Lasers: The unique energy levels within noble gas atoms contribute to their use in lasers, where precise control over ionization is critical.
- Inert Atmospheres: The inert nature of noble gases, stemming from their high ionization energies, makes them suitable for creating inert atmospheres in various industrial processes where the prevention of oxidation or other reactions is vital.
Advanced Concepts:
The ionization energy of noble gases is not just a simple number; it's a fundamental property deeply rooted in quantum mechanics. Factors like electron configuration, orbital shape, and spin-orbit coupling significantly influence the precise values of ionization energies. Advanced computational methods like Density Functional Theory (DFT) and Coupled Cluster (CC) calculations are employed to accurately predict and understand these values. These calculations take into account the intricate interactions between electrons and the nucleus, providing a detailed picture of the atomic structure and its impact on ionization energy.
Conclusion:
Noble gases possess exceptionally high ionization energies due to their complete valence electron shells, making them exceptionally stable and unreactive. While a general trend of decreasing ionization energy down the group is observed, subtle deviations exist due to the complex interplay of nuclear charge, shielding, and inter-electronic repulsion. The high ionization energies of noble gases have critical implications across various scientific and technological fields, highlighting their unique position in the periodic table. Further research continues to refine our understanding of these properties, delving deeper into the intricate quantum mechanical principles that govern atomic behavior and ionization processes. Understanding these principles allows for the development of new technologies and applications based on the exceptional properties of noble gases. The exploration of ionization energy remains a cornerstone of chemical physics, providing valuable insights into the fundamental nature of matter.
Latest Posts
Latest Posts
-
Analyze How Meiosis Produces Haploid Gametes
Mar 23, 2025
-
What Are Two Kinds Of Pure Substances
Mar 23, 2025
-
What Is The Smallest Particle In An Element
Mar 23, 2025
-
Gas Laws Practice Problems And Answers
Mar 23, 2025
-
Is Carbonate Ion A Strong Base
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Do Noble Gases Have Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
