Do Protons Have The Same Mass As Neutrons
Muz Play
Apr 07, 2025 · 5 min read
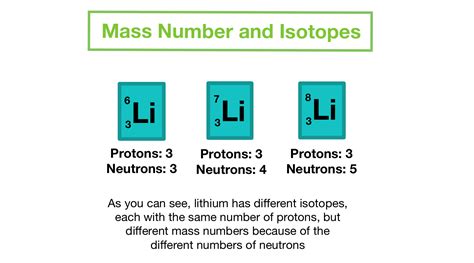
Table of Contents
Do Protons Have the Same Mass as Neutrons? A Deep Dive into Subatomic Particles
The seemingly simple question of whether protons and neutrons have the same mass opens a fascinating window into the intricacies of nuclear physics. While often treated as having similar masses in introductory physics, a closer examination reveals a subtle but significant difference. Understanding this difference requires delving into the composition of these particles, their interactions, and the implications for various physical phenomena.
The Standard Model and the Composition of Protons and Neutrons
At the heart of understanding the mass difference lies the Standard Model of particle physics. This model describes the fundamental building blocks of matter as quarks and leptons, governed by fundamental forces mediated by gauge bosons. Protons and neutrons, collectively known as nucleons, are not fundamental particles themselves; instead, they are composite particles made up of quarks.
Quarks: The Constituents of Nucleons
Protons and neutrons are each composed of three quarks bound together by the strong force, mediated by gluons. The specific quark composition distinguishes protons from neutrons:
- Proton: Two up quarks (u) and one down quark (d) – denoted as uud.
- Neutron: One up quark (u) and two down quarks (d) – denoted as udd.
The difference in quark composition is directly responsible for the slight mass discrepancy between protons and neutrons. However, it's not simply a matter of adding up the individual quark masses. The strong force binding the quarks together contributes significantly to the overall mass of the nucleon, a phenomenon known as quark confinement.
Mass Difference: A Subtle but Significant Variation
While the mass difference might seem insignificant at first glance, it plays a crucial role in various nuclear processes. The mass of a proton is approximately 1.007276 atomic mass units (amu), while the mass of a neutron is approximately 1.008665 amu. This translates to a neutron being about 0.14% more massive than a proton.
The Role of Binding Energy
The strong force, responsible for holding the quarks together within nucleons, also contributes to the overall mass. The energy required to separate the quarks is immense, and according to Einstein's famous equation, E=mc², this energy contributes to the mass of the nucleon. The difference in the binding energies of protons and neutrons due to their different quark compositions also contributes to the observed mass difference.
Implications of the Mass Difference
The seemingly small mass difference between protons and neutrons has profound consequences in various areas of physics:
Nuclear Stability and Isotopes
The mass difference is a crucial factor in determining the stability of atomic nuclei. The ratio of protons to neutrons in a nucleus significantly impacts its stability. Too many neutrons relative to protons can lead to beta-minus decay, where a neutron transforms into a proton, an electron, and an antineutrino. Conversely, too many protons relative to neutrons can lead to beta-plus decay or electron capture. This balance is delicately determined by the interplay of the strong and weak forces and is influenced by the slight mass difference between protons and neutrons. Isotopes of the same element differ in their neutron count, leading to variations in stability and other properties.
Nuclear Reactions and Fission
The mass difference plays a critical role in nuclear reactions such as fission and fusion. In nuclear fission, the splitting of a heavy nucleus into lighter nuclei releases energy because the binding energy per nucleon is higher in the lighter nuclei. This energy release is partly due to the mass difference between protons and neutrons influencing the overall binding energy. Similarly, in nuclear fusion, the combination of lighter nuclei into heavier ones releases energy because of the changes in binding energy, again linked to the mass difference between protons and neutrons.
Particle Physics Experiments
The slight mass difference between protons and neutrons is a significant factor in various particle physics experiments. Precise measurements of the masses of protons and neutrons are crucial for testing theoretical models of the strong force and the Standard Model. Discrepancies between experimental measurements and theoretical predictions can point towards new physics beyond the Standard Model. High-energy particle accelerators are used to probe the fundamental interactions of protons and neutrons at very high energies, where the mass difference, though small, plays a crucial role in the collision dynamics.
Beyond the Basic Model: Refining Our Understanding
While the basic quark model provides a good understanding of the mass difference, a more nuanced picture requires considering other factors:
Quantum Chromodynamics (QCD)
QCD is the theory that describes the strong force and the interactions of quarks and gluons. Precise calculations within QCD are computationally challenging, but they provide increasingly accurate predictions of the masses of protons and neutrons. These calculations account for the complex interactions between quarks and gluons and contribute to a deeper understanding of the mass difference.
Quantum Fluctuations
Quantum fluctuations of the vacuum create virtual particle-antiparticle pairs that briefly exist and then annihilate. These fluctuations contribute to the effective masses of protons and neutrons, further influencing the slight difference between them. These contributions, although subtle, need to be considered for high-precision calculations.
Conclusion: A Complex Interplay of Forces
The question of whether protons and neutrons have the same mass is not a simple yes or no. While they have remarkably similar masses, a subtle but significant difference exists, stemming from their differing quark compositions and the complexities of the strong force. This seemingly small difference has profound implications for nuclear stability, nuclear reactions, and the very structure of matter as we understand it. Further research and refinement of our theoretical models continue to shed light on this fascinating aspect of particle physics, pushing the boundaries of our understanding of the fundamental forces that govern the universe. The slight difference in mass between protons and neutrons is not just a numerical curiosity but a key component in the intricate tapestry of nuclear physics, a testament to the elegance and complexity of the universe at its most fundamental level. Continuing exploration and experimental refinement will undoubtedly lead to an even deeper understanding of this fundamental aspect of the physical world.
Latest Posts
Latest Posts
-
What Characteristics Do Ecologists Study To Learn About Populations
Apr 08, 2025
-
Examples Of First Order Linear Differential Equations
Apr 08, 2025
-
Van Der Waals Vs Hydrogen Bonds
Apr 08, 2025
-
How To Find The Orthogonal Basis
Apr 08, 2025
-
Is Sodium A Substance Or Mixture
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Do Protons Have The Same Mass As Neutrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.