Do Valence Electrons Have The Most Energy
Muz Play
Mar 17, 2025 · 5 min read
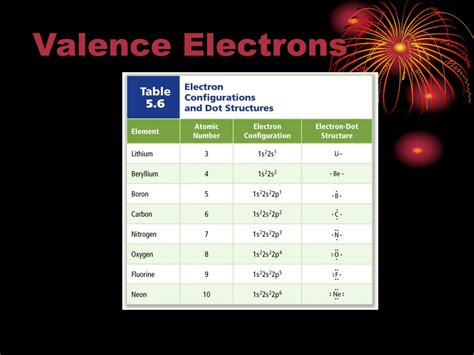
Table of Contents
Do Valence Electrons Have the Most Energy? Understanding Electron Energy Levels
The question of whether valence electrons possess the most energy within an atom is a common one, and the answer is nuanced. While valence electrons are crucial for chemical bonding and reactivity, their energy isn't necessarily the highest in the atom. Understanding this requires a deeper dive into atomic structure and electron configuration.
Atomic Structure and Electron Shells
Atoms consist of a nucleus containing protons and neutrons, surrounded by a cloud of electrons. These electrons are not randomly distributed; they occupy specific energy levels or shells, often depicted as concentric circles around the nucleus. These shells are designated by principal quantum numbers (n), with n=1 representing the shell closest to the nucleus (the lowest energy level), n=2 the next shell, and so on. Each shell can hold a maximum number of electrons, determined by the formula 2n².
Subshells and Orbitals
Within each shell, electrons further occupy subshells, denoted by letters (s, p, d, f). Each subshell contains one or more orbitals, which are regions of space where there's a high probability of finding an electron. The s subshell has one orbital, the p subshell has three orbitals, the d subshell five, and the f subshell seven. Each orbital can hold a maximum of two electrons, according to the Pauli Exclusion Principle.
Key takeaway: Electrons closer to the nucleus experience a stronger attractive force from the positively charged protons and therefore have lower energy. Electrons in outer shells experience a weaker attraction and possess higher energy.
Valence Electrons: The Outermost Players
Valence electrons are the electrons located in the outermost shell of an atom. These are the electrons most involved in chemical reactions and the formation of chemical bonds. Their relative ease of participation in bonding stems from their higher energy compared to inner electrons. They are less tightly bound to the nucleus and require less energy to be removed or shared.
Why Valence Electrons are Important for Reactivity
The number of valence electrons determines an element's chemical properties and its position in the periodic table. Elements in the same group (vertical column) have the same number of valence electrons and exhibit similar chemical behavior. For instance, the alkali metals (Group 1) all have one valence electron, making them highly reactive. Noble gases (Group 18), with their full valence shells, are largely inert.
Energy Levels and Ionization Energy
The energy required to remove an electron from an atom is called ionization energy. The first ionization energy is the energy needed to remove the first electron, the second ionization energy to remove the second, and so on. Generally, valence electrons have lower ionization energies than inner electrons. This is because they experience less attraction from the nucleus and are therefore easier to remove. However, this does not automatically mean they possess the highest energy within the atom.
Comparing Valence Electron Energy to Inner Electrons
While valence electrons have higher energy than inner electrons in the same atom, this doesn't necessarily mean they have the absolute highest energy possible within the atom. Consider the following:
-
Excited States: When an atom absorbs energy, an electron can jump to a higher energy level, potentially exceeding the energy of a valence electron in its ground state. This creates an excited state. In such a state, an inner electron promoted to a higher energy level will have more energy than the valence electrons.
-
Penetration and Shielding: The effect of inner electrons shielding the outer electrons from the full nuclear charge is crucial. An electron in a lower shell partially screens the nuclear charge, reducing the effective nuclear charge felt by valence electrons. Therefore, while a valence electron might have higher energy than an inner electron in the same shell, it's not always the case for electrons in different subshells within the same shell. For example, a 3d electron generally has slightly lower energy than a 4s electron, despite the 4s electron being in a higher principal quantum shell (and further from the nucleus). This is due to the greater penetration of the 3d orbitals compared to 4s orbitals.
-
Quantum Mechanical Considerations: A precise, single energy value for each electron is impossible to assign due to the probabilistic nature of quantum mechanics. Electrons exist in orbitals with associated energy probability distributions, not fixed energy levels. It is more accurate to discuss ranges of energy for different subshells within the same shell.
The Role of Effective Nuclear Charge
The effective nuclear charge (Zeff) is the net positive charge experienced by an electron after the shielding effect of other electrons has been accounted for. Valence electrons experience a reduced Zeff compared to inner electrons, making them less tightly bound to the nucleus. However, the difference in Zeff between valence electrons and inner electrons is not always a simple linear relationship, especially in multi-electron atoms.
Conclusion: A More Nuanced Perspective
To definitively state that valence electrons always have the most energy is incorrect. While they generally have higher energy than core electrons due to their distance from the nucleus and the shielding effect, this is not universally true. Excited states, penetration effects, and the complexities of quantum mechanics introduce nuances that make a simple "yes" or "no" answer insufficient.
Valence electrons are critical for chemical reactivity and bonding because of their relatively high energy and loose association with the nucleus. However, the energy landscape of an atom is more intricate than a simple ranking of electron energies based solely on shell number. Understanding the concepts of effective nuclear charge, electron shielding, subshell energies, and excited states provides a more complete picture of the energetic behavior of electrons within an atom. The focus should be on the comparative energy of valence electrons relative to other electrons within the same atom in its ground state, rather than claiming they are always the most energetic electrons overall.
Latest Posts
Latest Posts
-
Name A Structural Difference Between Triglycerides And Phospholipids
Mar 17, 2025
-
Ending Materials In A Chemical Reaction
Mar 17, 2025
-
Get Energy By Eating Other Organisms
Mar 17, 2025
-
What Is A Power Function In Math
Mar 17, 2025
-
How Many Phosphate Groups Does Atp Have
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do Valence Electrons Have The Most Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
