Does A Double Bond Count As One Electron Group
Muz Play
Mar 21, 2025 · 5 min read
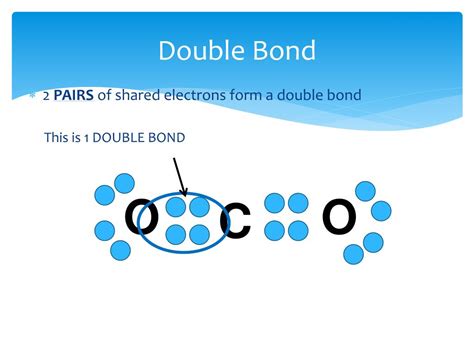
Table of Contents
Does a Double Bond Count as One Electron Group? Understanding VSEPR Theory
When delving into the world of molecular geometry and predicting the shapes of molecules, understanding electron group arrangements is paramount. The Valence Shell Electron Pair Repulsion (VSEPR) theory provides a framework for this prediction, relying on the fundamental principle that electron groups—whether bonding pairs or lone pairs—repel each other and arrange themselves to maximize the distance between them. A key question that often arises, particularly for students new to chemistry, is: does a double bond count as one electron group? The answer is yes, but understanding why requires a deeper dive into the theory itself.
Understanding Electron Groups and VSEPR Theory
VSEPR theory is a simple yet powerful model that predicts the three-dimensional arrangement of atoms in a molecule based on the electron pairs surrounding the central atom. These electron pairs, whether involved in bonding or existing as lone pairs, are considered electron groups. It's crucial to remember that the VSEPR model considers the number of electron groups, not the number of individual electrons.
The core principle behind VSEPR is that electron groups repel each other due to their negative charge. This repulsion forces them to arrange themselves as far apart as possible to minimize the energy of the molecule. This arrangement determines the overall molecular geometry.
Types of Electron Groups
Before we address double bonds, let's clarify the types of electron groups:
- Bonding Pairs: These are electron pairs shared between two atoms to form a covalent bond (single, double, or triple). Each bond, regardless of its type, contributes one electron group.
- Lone Pairs: These are electron pairs that are not involved in bonding. They are associated with the central atom and contribute one electron group each.
The Case of Double Bonds: Why They Count as One Electron Group
A double bond consists of two pairs of electrons shared between two atoms. While it involves two pairs of electrons, they occupy the same region of space between the two bonded atoms. They are not spatially distinct electron groups like lone pairs or the electron pairs in a single bond. The electron density is concentrated between the atoms, creating one region of repulsion.
Think of it this way: Imagine two balloons tied together at a single point. While there are two balloons (electron pairs), they act as a single unit of repulsion, occupying the same general space. This is analogous to a double bond: both electron pairs occupy the same region of space between the bonded atoms, contributing to a single electron group.
Similarly, a triple bond also counts as one electron group because all three pairs of electrons reside within the same spatial region between the two bonded atoms.
Predicting Molecular Geometry Using VSEPR
The number of electron groups dictates the electron-group geometry, while the number of lone pairs influences the molecular geometry. Let's look at some examples:
Example 1: Carbon Dioxide (CO₂)
CO₂ has a central carbon atom double-bonded to two oxygen atoms. Each double bond contributes one electron group, resulting in a total of two electron groups. The electron-group geometry and molecular geometry are both linear.
Example 2: Ethene (C₂H₄)
In ethene, each carbon atom has three electron groups: one double bond to the other carbon atom and two single bonds to hydrogen atoms. Around each carbon, the electron-group geometry is trigonal planar, and the molecular geometry is also trigonal planar.
Example 3: Water (H₂O)
Water has a central oxygen atom with two single bonds to hydrogen atoms and two lone pairs. This gives a total of four electron groups. The electron-group geometry is tetrahedral, but the presence of two lone pairs results in a bent molecular geometry.
Common Misconceptions about Double Bonds and VSEPR
It's essential to address common misconceptions related to double bonds and VSEPR theory:
- Misconception 1: Double bonds contribute two electron groups because they have two pairs of electrons. Correction: While a double bond has two pairs of electrons, they occupy the same spatial region, acting as a single unit of repulsion and hence contributing only one electron group.
- Misconception 2: The VSEPR model considers individual electrons. Correction: VSEPR considers electron groups, not individual electrons. Each bonding pair or lone pair represents one electron group, irrespective of the number of electrons within that group.
Advanced Considerations: Bond Order and Electron Density
While VSEPR treats double and triple bonds as single electron groups for geometry prediction, it's important to acknowledge that the higher bond order affects electron density. A double bond has a higher electron density between the atoms than a single bond, resulting in slightly stronger repulsion. This subtle difference is not explicitly accounted for in basic VSEPR applications but might influence bond lengths and angles to a small extent. More sophisticated models are needed to analyze these finer details.
Conclusion: Applying the Principle in Practice
Understanding the fact that a double bond counts as one electron group is fundamental to successfully applying VSEPR theory. This principle simplifies the process of predicting molecular geometries, allowing us to visualize the three-dimensional arrangement of atoms and understand the properties of various molecules. By accurately identifying the number of electron groups, we can effectively predict shapes and consequently, understand many molecular properties. Remember the core principle: the number of electron groups determines the overall shape, not the individual electron count within each bond. Mastering this concept is crucial for success in chemistry studies and for further explorations in more complex chemical phenomena. Keep practicing and building your understanding of the VSEPR model and you'll find molecular geometry prediction becomes second nature.
Latest Posts
Latest Posts
-
Mannitol Salt Agar Is Selective For
Mar 21, 2025
-
What Is The Cleft Of Venus
Mar 21, 2025
-
Describe Internal Factors Of Decision Making
Mar 21, 2025
-
How Does Thermal Energy Affect The 3 States Of Matter
Mar 21, 2025
-
Is Solubility A Chemical Or Physical Property
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Does A Double Bond Count As One Electron Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
