Does Calcium Lose Or Gain Electrons
Muz Play
Mar 17, 2025 · 5 min read
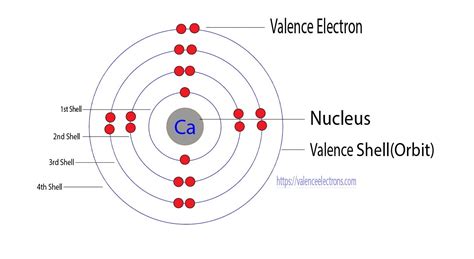
Table of Contents
Does Calcium Lose or Gain Electrons? Understanding Calcium's Electron Behavior
Calcium, a vital element for human health and a common component in many materials, plays a fascinating role in the world of chemistry. A key aspect of understanding calcium's behavior lies in its electron configuration and how it interacts with other elements. This article delves into the intricacies of calcium's electron behavior, exploring whether it loses or gains electrons and the fundamental principles governing this process. We'll also explore the implications of this electron behavior in various chemical reactions and biological processes.
Calcium's Position in the Periodic Table: A Crucial Clue
Before diving into the specifics of electron gain or loss, let's consider calcium's location on the periodic table. Calcium (Ca) is an alkaline earth metal, residing in Group 2, Period 4. This placement provides crucial insights into its electronic structure and reactivity. Group 2 elements are characterized by having two electrons in their outermost shell, also known as the valence shell. This electron configuration is the key to understanding calcium's tendency to lose or gain electrons.
Valence Electrons: The Key Players
The valence electrons are the electrons furthest from the nucleus and are most easily involved in chemical bonding. These electrons determine an element's chemical properties and reactivity. For calcium, these two valence electrons are relatively loosely held compared to the inner electrons. This loose binding makes them prone to interaction and participation in chemical reactions.
Why Calcium Loses Electrons: The Octet Rule
The driving force behind calcium's behavior is the octet rule, a fundamental principle in chemistry. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost shell, resembling the electron configuration of noble gases.
Calcium, with its two valence electrons, can achieve a stable octet by losing these two electrons. By doing so, it attains the same electron configuration as the noble gas Argon (Ar), which has a full outer shell of eight electrons. This stable configuration is energetically favorable, meaning it is a lower energy state than having two valence electrons. Therefore, calcium readily gives up its valence electrons to become more stable.
Ionization Energy and Electron Loss
The energy required to remove an electron from a neutral atom is called ionization energy. Calcium has relatively low ionization energies for its first and second electrons. This low ionization energy indicates that removing these two valence electrons requires comparatively little energy. Once these two electrons are removed, the resulting ion has a significantly higher ionization energy, making it difficult to remove any further electrons.
Calcium's Formation of Ions: Ca²⁺
When calcium loses its two valence electrons, it becomes a positively charged ion, denoted as Ca²⁺. This ion is known as a cation, because it carries a positive charge. The loss of negatively charged electrons results in a net positive charge on the atom. This cationic form of calcium is exceptionally stable due to its complete octet.
Chemical Reactions and Electron Transfer
Calcium's tendency to lose electrons is evident in its participation in various chemical reactions. These reactions often involve electron transfer from calcium to another atom or molecule with a higher electronegativity. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond.
Reaction with Nonmetals: An Example
A classic example is the reaction of calcium with chlorine (Cl₂). Chlorine, being a highly electronegative element, readily accepts electrons. In this reaction, calcium loses its two valence electrons, which are accepted by two chlorine atoms, each gaining one electron. This electron transfer leads to the formation of calcium chloride (CaCl₂), an ionic compound held together by electrostatic attraction between the positively charged Ca²⁺ ions and the negatively charged Cl⁻ ions.
Ca + Cl₂ → CaCl₂
This reaction is a prime example of an oxidation-reduction reaction, or redox reaction. Calcium is oxidized (loses electrons) and chlorine is reduced (gains electrons).
Biological Significance of Calcium's Electron Behavior
Calcium's ability to lose electrons and form stable cations plays a crucial role in numerous biological processes. The Ca²⁺ ion is an essential component in various biological systems, including:
-
Bone and Teeth Formation: Calcium ions are vital for the formation and maintenance of strong bones and teeth. They combine with phosphate ions to create the mineral hydroxyapatite, the primary structural component of bone.
-
Muscle Contraction: Calcium ions are crucial for muscle contraction. The release of Ca²⁺ ions from intracellular stores triggers the interaction between actin and myosin filaments, leading to muscle contraction.
-
Nerve Impulse Transmission: Calcium ions also play a crucial role in nerve impulse transmission. The influx of Ca²⁺ ions into nerve cells facilitates the release of neurotransmitters, enabling communication between nerve cells.
-
Blood Clotting: Calcium ions are essential cofactors in several steps of the blood clotting cascade. They contribute to the activation of various clotting factors, ultimately leading to the formation of a blood clot.
Conclusion: Calcium's Electron Loss Defines its Reactivity
In conclusion, calcium consistently loses electrons in chemical reactions, rather than gaining them. This characteristic behavior stems directly from its electron configuration and the drive to achieve a stable octet electron arrangement. This electron loss leads to the formation of the stable Ca²⁺ ion, a crucial player in various chemical and biological processes. Understanding calcium's electron behavior is fundamental to comprehending its reactivity and importance in both the natural world and various applications. The low ionization energy of its valence electrons makes the loss of electrons an energetically favorable process, defining calcium's role as a readily reactive alkaline earth metal. This fundamental property shapes its contributions to everything from the strength of our bones to the intricate workings of our nervous system.
Latest Posts
Latest Posts
-
Corn Kernel Positive Or Negative Gravitropism
Mar 17, 2025
-
Angle Properties Of A Circle Outside The Circle
Mar 17, 2025
-
Como Se Separa En Silabas La Palabra Tienda
Mar 17, 2025
-
Tube Open At Both Ends Harmonic Equation
Mar 17, 2025
-
A Shaft Of A Long Bone Is Called
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Calcium Lose Or Gain Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
