Electrons Are Bigger Than Protons And Neutrons
Muz Play
Mar 18, 2025 · 5 min read
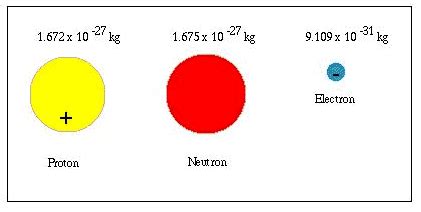
Table of Contents
Electrons are Bigger than Protons and Neutrons: A Misconception Debunked
The statement "electrons are bigger than protons and neutrons" is a common misconception. While seemingly counterintuitive, it's crucial to understand that the size of subatomic particles isn't as straightforward as it might first appear. The truth is far more nuanced and involves a deeper understanding of quantum mechanics and the nature of particle physics. This article will delve into the complexities of subatomic particle size, debunking the misconception and clarifying the true nature of protons, neutrons, and electrons.
The Problem with Defining "Size" in the Quantum Realm
The difficulty in comparing the sizes of protons, neutrons, and electrons stems from the fact that these particles aren't solid, billiard-ball-like objects. Instead, they are governed by the laws of quantum mechanics, which dictate that their properties are probabilistic rather than deterministic. We can't simply measure their diameter using a ruler.
Instead, we need to consider different ways to define size:
1. The Classical Radius: A Limited Perspective
One attempt to define size is through the classical electron radius, which is calculated based on the electron's charge and mass, assuming it's a uniformly charged sphere. Similarly, a classical radius can be calculated for protons and neutrons. However, this approach is inherently flawed because it ignores the quantum mechanical nature of these particles. It's a model based on classical physics and doesn't accurately reflect reality.
2. The Root-Mean-Square Charge Radius: A More Accurate Representation
A more sophisticated and accurate method is to determine the root-mean-square (RMS) charge radius. This approach considers the probability distribution of the particle's charge. The RMS charge radius gives us a measure of the average extent of the charge distribution.
For protons, the RMS charge radius is approximately 0.877 femtometers (fm), where 1 fm = 10<sup>-15</sup> meters. Neutrons, being electrically neutral, have a more complex charge distribution which makes determining a comparable radius more difficult, but it's similarly on the order of femtometers.
Electrons, however, pose a different challenge. Because electrons are fundamental particles, meaning they're not composed of smaller constituents, they don't have a well-defined size in the same way that protons and neutrons do. They're often described as point particles, meaning they have no spatial extent. While various theoretical models might assign an effective size to an electron, these are purely theoretical constructions and not measurements of an actual physical dimension.
The Crucial Difference: Composition and Structure
The key difference between electrons and protons/neutrons lies in their composition:
-
Electrons: These are fundamental particles, belonging to the lepton family. They are elementary particles, meaning they are not made up of smaller constituents.
-
Protons and Neutrons: These are composite particles, meaning they are made up of smaller constituents called quarks. Protons contain two up quarks and one down quark, while neutrons contain one up quark and two down quarks. These quarks are held together by the strong force, mediated by gluons. The complex interactions between these constituent particles give protons and neutrons their size and properties.
Comparing Sizes: A Matter of Scale and Perspective
The size of a proton or neutron is determined by the extent of the quark confinement. While it's not a simple matter of measuring a solid sphere, the RMS charge radius provides a useful measure. Given the size of a proton (approximately 0.877 fm), and the size of a neutron being comparable, it's clear that they are significantly larger than the (effectively) zero size of an electron.
To further illustrate the scale involved, let's imagine scaling up these particles:
If a proton were scaled up to the size of a grapefruit, an electron would be smaller than the period at the end of this sentence. This comparison highlights the immense difference in scale between these particles.
The Role of Quantum Mechanics: Uncertainty and Probability
The quantum mechanical nature of these particles adds another layer of complexity. The Heisenberg uncertainty principle states that we cannot simultaneously know both the position and momentum of a particle with perfect accuracy. This means that the concept of a precisely defined boundary becomes blurred in the quantum realm. Instead, we talk about probability distributions: the likelihood of finding a particle within a certain volume.
For electrons, this probability distribution is spread out over a larger area compared to the confined space occupied by the quarks within a proton or neutron. However, it's critical to understand that this doesn't mean the electron is physically "larger" in terms of a solid volume. It simply reflects the nature of quantum mechanical probability.
Misconceptions and Their Origins
The misconception that electrons are bigger than protons and neutrons likely stems from several factors:
-
Electron Clouds in Atomic Models: Simple atomic models often depict electrons as orbiting the nucleus in distinct shells or clouds. This visual representation can mislead individuals into thinking that these clouds represent the actual physical size of the electron.
-
The Wave-Particle Duality: The wave-particle duality of electrons further complicates the understanding of their size. Electrons exhibit both wave-like and particle-like properties, which adds to the difficulty of visualizing their physical dimensions.
-
Lack of Precise Measurement: The inherent difficulty in directly measuring the size of an electron using classical methods contributes to the ambiguity.
Conclusion: A Clearer Understanding of Subatomic Particles
In conclusion, the assertion that electrons are bigger than protons and neutrons is inaccurate. While electrons have a probability distribution that can be described by a wave function, this does not equate to them having a larger physical size than protons or neutrons. Protons and neutrons, being composite particles made of quarks, have a well-defined physical extent (although not in the classical sense), whereas electrons are more accurately described as point particles. The seemingly paradoxical nature of subatomic particle size highlights the limitations of classical physics in understanding the quantum world. A deep understanding of quantum mechanics is necessary to grasp the true nature of these fundamental building blocks of matter. The misconception stems from simplified models and a lack of appreciation for the probabilistic nature of quantum mechanics. Understanding the true nature of these particles requires embracing the complexities of quantum theory and rejecting simplistic analogies based on classical notions of size and volume.
Latest Posts
Latest Posts
-
What Is The Difference Between Primary And Secondary Growth
Mar 18, 2025
-
Work Done By An Electric Field
Mar 18, 2025
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Electrons Are Bigger Than Protons And Neutrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
