Electrons In An Atom's Outermost Energy Shells Are Called
Muz Play
Mar 25, 2025 · 7 min read
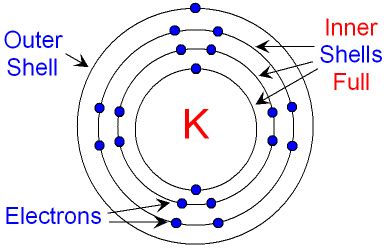
Table of Contents
Electrons in an Atom's Outermost Energy Shells are Called Valence Electrons: Understanding Their Crucial Role in Chemistry
Electrons, those fundamental negatively charged particles whizzing around the nucleus of an atom, don't all play the same role. Their behavior, particularly those residing in the outermost energy shell, dictates an atom's chemical properties and reactivity. These crucial electrons are called valence electrons. Understanding valence electrons is fundamental to grasping the principles of chemical bonding, molecular structure, and the behavior of matter itself. This article delves deep into the world of valence electrons, exploring their significance in various chemical phenomena.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell or energy level of an atom. This outermost shell is often referred to as the valence shell. These electrons are the most loosely bound to the nucleus and are therefore most readily involved in chemical reactions. They determine an atom's ability to form chemical bonds with other atoms, dictating its reactivity and the types of compounds it can form. The number of valence electrons an atom possesses directly influences its chemical behavior.
Understanding Electron Shells and Subshells
Before we dive deeper into valence electrons, it's important to understand the organization of electrons within an atom. Electrons are arranged in distinct energy levels or shells, each capable of holding a specific number of electrons. These shells are designated by principal quantum numbers (n = 1, 2, 3, etc.), with n=1 representing the shell closest to the nucleus. Within each shell, there are subshells (s, p, d, f) that further define the electron's energy and spatial distribution.
- Shell 1 (n=1): Contains only one subshell, the 1s subshell, which can hold a maximum of 2 electrons.
- Shell 2 (n=2): Contains two subshells: the 2s (2 electrons) and 2p (6 electrons) subshells, for a total of 8 electrons.
- Shell 3 (n=3): Contains three subshells: 3s (2 electrons), 3p (6 electrons), and 3d (10 electrons), allowing for a maximum of 18 electrons.
- Higher Shells (n>3): Follow a similar pattern, with increasing numbers of subshells and a correspondingly higher electron capacity.
The filling of these shells and subshells follows specific rules, primarily the Aufbau principle and Hund's rule, which dictate the order in which electrons occupy available orbitals. However, for understanding valence electrons, it's the outermost occupied shell that holds the most importance.
Determining the Number of Valence Electrons
Determining the number of valence electrons for an atom is crucial for predicting its chemical behavior. There are several ways to do this:
1. Using the Periodic Table
The periodic table is a powerful tool for determining the number of valence electrons. The group number (vertical column) of an element in the periodic table generally corresponds to the number of valence electrons. For example:
- Group 1 (Alkali Metals): 1 valence electron
- Group 2 (Alkaline Earth Metals): 2 valence electrons
- Group 13 (Boron Group): 3 valence electrons
- Group 14 (Carbon Group): 4 valence electrons
- Group 15 (Pnictogens): 5 valence electrons
- Group 16 (Chalcogens): 6 valence electrons
- Group 17 (Halogens): 7 valence electrons
- Group 18 (Noble Gases): 8 valence electrons (except Helium, which has 2)
Note: This rule applies primarily to the main group elements (s and p blocks). Transition metals (d block) and inner transition metals (f block) have more complex valence electron configurations.
2. Using Electron Configuration
The electron configuration of an atom shows the distribution of electrons in its various shells and subshells. By examining the electron configuration, we can identify the number of electrons in the outermost shell, which represents the valence electrons. For example, the electron configuration of oxygen (O) is 1s²2s²2p⁴. The outermost shell is the second shell (n=2), containing 2+4=6 electrons. Therefore, oxygen has 6 valence electrons.
3. Using Lewis Dot Structures
Lewis dot structures are simplified representations of atoms and molecules that show the valence electrons as dots surrounding the element's symbol. These structures are particularly useful for visualizing the participation of valence electrons in chemical bonding.
The Significance of Valence Electrons in Chemical Bonding
Valence electrons are the primary players in chemical bonding, the forces that hold atoms together in molecules and compounds. Atoms tend to react in ways that achieve a stable electron configuration, often resembling that of a noble gas (8 valence electrons, or 2 for Helium). This tendency is the basis of the octet rule.
There are several types of chemical bonds, all involving the interaction of valence electrons:
1. Ionic Bonding
Ionic bonding occurs when one atom transfers one or more valence electrons to another atom. This transfer creates ions: positively charged cations (atom that loses electrons) and negatively charged anions (atom that gains electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond. For example, in sodium chloride (NaCl), sodium (Na) loses one valence electron to chlorine (Cl), forming Na⁺ and Cl⁻ ions, which are held together by ionic bonds.
2. Covalent Bonding
Covalent bonding involves the sharing of valence electrons between atoms. Atoms share electrons to achieve a stable electron configuration. The shared electrons are attracted to the nuclei of both atoms, forming a covalent bond. For example, in methane (CH₄), carbon shares its four valence electrons with four hydrogen atoms, each contributing one electron to form four covalent bonds.
3. Metallic Bonding
Metallic bonding occurs in metals, where valence electrons are delocalized and form a "sea" of electrons surrounding positively charged metal ions. This "sea" of electrons allows for the high electrical and thermal conductivity characteristic of metals.
Valence Electrons and Chemical Reactivity
The number of valence electrons significantly influences an atom's chemical reactivity. Atoms with nearly full valence shells (e.g., halogens with 7 valence electrons) are highly reactive, readily gaining an electron to achieve a stable octet. Atoms with few valence electrons (e.g., alkali metals with 1 valence electron) are also highly reactive, readily losing an electron to achieve a stable configuration. Atoms with 4 valence electrons (e.g., carbon) exhibit a variety of bonding patterns, forming diverse compounds. Noble gases, with their full valence shells, are exceptionally unreactive.
Valence Electrons and Periodicity
The periodic repetition of chemical properties across the periodic table is directly linked to the periodic variation in the number of valence electrons. Elements within the same group (vertical column) have the same number of valence electrons, leading to similar chemical behaviors. This periodicity is a cornerstone of chemistry, allowing us to predict and understand the properties of elements based on their position in the periodic table.
Beyond the Octet Rule: Exceptions and Complications
While the octet rule provides a useful framework for understanding chemical bonding, it has exceptions. Some atoms can have fewer than eight valence electrons (e.g., boron in BF₃) or more than eight (e.g., phosphorus in PF₅). These exceptions arise from factors such as the availability of d orbitals for bonding and the relative strengths of different types of bonds.
Applications of Understanding Valence Electrons
Understanding valence electrons is crucial in numerous applications across various scientific fields:
- Materials Science: Designing new materials with specific properties, such as conductivity, strength, or reactivity, often involves tailoring the number and arrangement of valence electrons.
- Catalysis: Catalysts, substances that speed up chemical reactions, often function by interacting with the valence electrons of reactant molecules.
- Drug Design: Designing drugs often involves manipulating the interactions of valence electrons to target specific biological molecules.
- Semiconductor Technology: The electronic properties of semiconductors are directly related to the behavior of their valence electrons.
Conclusion
Valence electrons, those outermost electrons in an atom, are far more than just occupants of an atom's periphery. They are the key players in chemical reactions, dictating an atom's reactivity and bonding behavior. Their importance extends across various fields of science and technology, making a thorough understanding of valence electrons essential for anyone interested in chemistry and its applications. From understanding simple chemical bonds to designing advanced materials, the role of valence electrons remains paramount in shaping our world. Their influence is pervasive, underlining the fundamental importance of this seemingly simple concept in unraveling the complexities of the chemical universe.
Latest Posts
Latest Posts
-
How To Calculate The Potential Difference
Mar 25, 2025
-
Why Race Is A Social Construct
Mar 25, 2025
-
Why Is Prophase The Longest Phase
Mar 25, 2025
-
Tipos De Triangulos Segun Sus Angulos
Mar 25, 2025
-
Did The Swahili Coast Require Monsoons To Access
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Electrons In An Atom's Outermost Energy Shells Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
