Electrons In The Outermost Energy Level Are Called
Muz Play
Mar 30, 2025 · 7 min read
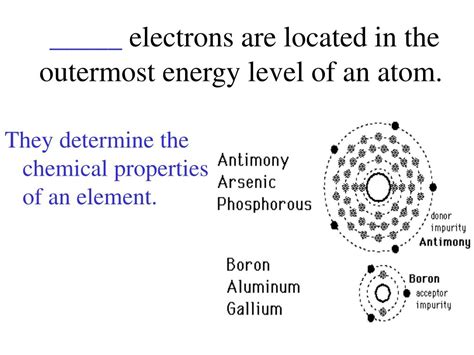
Table of Contents
Electrons in the Outermost Energy Level are Called Valence Electrons: Understanding Their Crucial Role in Chemistry
Electrons, the fundamental negatively charged particles orbiting an atom's nucleus, play a pivotal role in determining an element's chemical properties. Specifically, those residing in the outermost energy level, known as valence electrons, are the key players in chemical bonding and reactions. Understanding valence electrons is fundamental to grasping the intricacies of chemistry, from predicting the reactivity of elements to explaining the formation of complex molecules. This in-depth exploration will delve into the nature of valence electrons, their significance in chemical bonding, and their influence on various chemical phenomena.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell or energy level of an atom. This outermost shell is also often referred to as the valence shell. These electrons are the most loosely bound to the atom's nucleus and are therefore the most readily available to participate in chemical interactions. Their number dictates an element's reactivity and how it will bond with other atoms. Unlike the core electrons, which are closer to the nucleus and shielded by inner shells, valence electrons are directly involved in the formation of chemical bonds.
Determining the Number of Valence Electrons
The number of valence electrons an atom possesses can be readily determined using the element's position in the periodic table. The periodic table is organized in a way that reflects the electron configuration of the elements. Groups or columns on the periodic table represent elements with similar valence electron configurations.
- Group 1 (Alkali Metals): Elements in this group, such as lithium (Li), sodium (Na), and potassium (K), all have one valence electron.
- Group 2 (Alkaline Earth Metals): Elements in this group, such as beryllium (Be), magnesium (Mg), and calcium (Ca), all possess two valence electrons.
- Group 13 (Boron Group): Elements in this group have three valence electrons.
- Group 14 (Carbon Group): Elements in this group have four valence electrons.
- Group 15 (Pnictogens): Elements in this group have five valence electrons.
- Group 16 (Chalcogens): Elements in this group have six valence electrons.
- Group 17 (Halogens): Elements in this group have seven valence electrons.
- Group 18 (Noble Gases): Elements in this group, with the exception of helium (He), have eight valence electrons (a stable octet). This full valence shell contributes to their inertness.
Helium (He), a unique exception, has only two valence electrons, but this satisfies the duet rule (a stable configuration for the first energy level).
It's crucial to understand that the number of valence electrons is not directly equivalent to the total number of electrons in an atom. For example, oxygen (O) has a total of 8 electrons, but only 6 are valence electrons. The other two electrons occupy the inner energy level.
The Significance of Valence Electrons in Chemical Bonding
Valence electrons are the primary participants in the formation of chemical bonds, the forces that hold atoms together in molecules and compounds. Atoms strive to achieve a stable electron configuration, usually a full outer shell. This tendency drives chemical reactions and the formation of bonds.
Types of Chemical Bonds:
Several types of chemical bonds arise from the interaction of valence electrons:
-
Ionic Bonds: These bonds form when one atom transfers one or more valence electrons to another atom. This transfer results in the formation of ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. For example, in sodium chloride (NaCl), sodium (Na) loses one valence electron to chlorine (Cl), forming Na⁺ and Cl⁻ ions, which are then held together by an ionic bond.
-
Covalent Bonds: These bonds involve the sharing of valence electrons between two atoms. This sharing allows both atoms to achieve a more stable electron configuration. For instance, in a water molecule (H₂O), oxygen shares electrons with two hydrogen atoms, resulting in a stable configuration for all three atoms. Covalent bonds are particularly common in organic molecules and are responsible for the vast diversity of organic compounds.
-
Metallic Bonds: These bonds occur in metals, where valence electrons are delocalized and form a "sea" of electrons shared among all the metal atoms. This shared electron sea accounts for the characteristic properties of metals, such as their high electrical and thermal conductivity, malleability, and ductility.
Valence Electrons and Chemical Reactivity
The number of valence electrons significantly influences an element's chemical reactivity.
-
High Reactivity: Elements with nearly full or nearly empty valence shells (e.g., halogens with seven valence electrons and alkali metals with one valence electron) are highly reactive. They readily gain or lose electrons to achieve a stable electron configuration, leading to strong chemical bonds.
-
Low Reactivity: Elements with a full valence shell (noble gases) are extremely unreactive or inert. Their stable electron configuration means they have little tendency to gain, lose, or share electrons.
-
Variable Reactivity: Elements with intermediate numbers of valence electrons exhibit varying degrees of reactivity. Their behavior can depend on the specific conditions and other elements they are interacting with.
Valence Electrons and Oxidation States
The concept of oxidation states, a measure of the degree of oxidation (electron loss) or reduction (electron gain) of an atom in a compound, is closely related to valence electrons. The oxidation state of an atom reflects the number of electrons it has gained or lost compared to its neutral state. For instance, in NaCl, sodium has an oxidation state of +1 (having lost one electron), while chlorine has an oxidation state of -1 (having gained one electron). Understanding oxidation states is vital in balancing redox reactions and predicting the reactivity of compounds.
Valence Electrons and Molecular Geometry
The arrangement of atoms in a molecule (molecular geometry) is strongly influenced by the number and arrangement of valence electrons. The valence shell electron pair repulsion (VSEPR) theory provides a useful framework for predicting molecular shapes based on the number of electron pairs (both bonding and non-bonding) surrounding a central atom. The repulsion between electron pairs leads to specific geometries that minimize electron-electron interactions, influencing the molecule's overall properties.
Valence Electrons in Advanced Chemistry Concepts
The concept of valence electrons extends beyond basic chemical bonding and reactivity. It plays a critical role in several advanced chemical concepts:
-
Band Theory: In solids, especially metals and semiconductors, valence electrons form energy bands. The properties of these materials, including electrical conductivity, are directly related to the structure and occupancy of these bands.
-
Molecular Orbital Theory: This theory describes the formation of molecular orbitals from atomic orbitals, which are regions of space where valence electrons are most likely to be found. The resulting molecular orbitals determine the bonding characteristics of the molecule.
-
Coordination Chemistry: In coordination complexes, valence electrons of metal ions interact with ligands (molecules or ions bonded to the metal ion), forming coordination bonds. The properties of coordination complexes depend significantly on the number and nature of the valence electrons involved.
-
Spectroscopy: The interaction of light with matter often involves valence electrons. Techniques like UV-Vis spectroscopy and photoelectron spectroscopy directly probe the energy levels of valence electrons, providing information about the electronic structure of molecules and materials.
Conclusion
In summary, valence electrons are the cornerstone of understanding chemical behavior. Their number, arrangement, and participation in chemical bonds dictate an element's reactivity, bonding type, oxidation state, molecular geometry, and a multitude of other chemical properties. From simple ionic compounds to complex organic molecules and advanced materials, the influence of valence electrons is pervasive throughout chemistry. A thorough understanding of these outermost electrons is essential for anyone seeking to grasp the fundamentals and complexities of the chemical world. The periodic table serves as a powerful tool to quickly identify the number of valence electrons an element possesses, making it an invaluable resource for students and researchers alike. Further exploration of these concepts will undoubtedly reveal the profound impact valence electrons have on all aspects of chemical interactions and the properties of matter.
Latest Posts
Latest Posts
-
Foundations Of Education 13th Edition Pdf Free Download
Apr 01, 2025
-
Does Lead Need A Roman Numeral
Apr 01, 2025
-
Introduction To Chemical Reactions Answer Key
Apr 01, 2025
-
Is Melting An Ice Cube A Physical Or Chemical Change
Apr 01, 2025
-
Examples Of A Thesis Statement For A Literary Analysis
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Electrons In The Outermost Energy Level Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
