Energy Produced From The Movement Of Particles In A Substance
Muz Play
Mar 19, 2025 · 7 min read
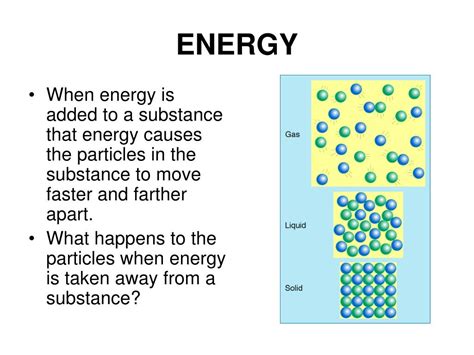
Table of Contents
Energy Produced from the Movement of Particles in a Substance: A Deep Dive into Thermal Energy
The world around us is a whirlwind of activity at the microscopic level. Atoms and molecules, the fundamental building blocks of matter, are in constant motion. This seemingly insignificant jiggling and vibrating is, in fact, the source of a significant form of energy: thermal energy, also known as heat energy. Understanding how this energy is produced, harnessed, and utilized is crucial to comprehending numerous scientific principles and technological advancements.
What is Thermal Energy?
Thermal energy is the kinetic energy associated with the random movement of atoms and molecules within a substance. This movement encompasses a wide range of motions, including translation (movement from one place to another), rotation (spinning around an axis), and vibration (oscillation around a fixed point). The faster these particles move, the higher the thermal energy of the substance. This energy is directly related to the substance's temperature: a higher temperature signifies a greater average kinetic energy of its constituent particles.
The Role of Temperature
Temperature isn't just a measure of how hot or cold something is; it's a quantitative indicator of the average kinetic energy of the particles. While temperature provides an overall picture, it doesn't reveal the complete story. Two substances at the same temperature can possess different amounts of thermal energy. A large swimming pool at 25°C holds significantly more thermal energy than a small cup of water at the same temperature because it contains vastly more particles.
The Link Between Particle Movement and Energy
The relationship between particle movement and energy is fundamental to understanding thermodynamics. Consider the following:
- Solids: In solids, particles are tightly packed and vibrate in fixed positions. Their movement is relatively restricted, resulting in lower thermal energy compared to liquids and gases.
- Liquids: Liquids have particles that are less tightly bound than in solids. They can move around more freely, leading to higher thermal energy and greater fluidity.
- Gases: Gases possess the highest thermal energy. Their particles are widely dispersed and move rapidly and randomly, colliding frequently with each other and the container walls.
This variation in particle movement directly affects the energy content of the substance. The energy transferred during these movements is what we experience as heat.
Harnessing Thermal Energy: From Natural Processes to Technology
The movement of particles isn't just a theoretical concept; it's the driving force behind many natural processes and technological applications. Let's explore some examples:
1. Geothermal Energy: Tapping into Earth's Internal Heat
The Earth's core is incredibly hot, generating vast amounts of thermal energy. This heat radiates outwards, warming the surrounding rocks and fluids. Geothermal energy harnesses this natural heat source. Deep underground, water is heated, often becoming superheated steam. This steam can be used to drive turbines, generating electricity in geothermal power plants. This process directly exploits the kinetic energy of the water molecules and the thermal energy transferred to them from the Earth's interior.
2. Solar Energy: Converting Sunlight into Usable Energy
Sunlight, the primary source of energy for our planet, is a manifestation of thermal energy. The sun's immense heat is the result of nuclear fusion, converting mass into energy. This energy travels to Earth as electromagnetic radiation, including visible light and infrared radiation. Solar panels utilize this energy by converting sunlight into electricity through the photovoltaic effect. While the mechanism is different from the direct conversion of particle movement, the underlying principle is the same: harnessing energy from the movement of particles (in this case, photons).
3. Fossil Fuels: Releasing Stored Solar Energy
Fossil fuels like coal, oil, and natural gas are essentially ancient stores of solar energy. Millions of years ago, plants captured sunlight through photosynthesis, converting it into chemical energy stored in their organic matter. When we burn these fuels, we are essentially releasing this stored energy. The chemical reactions involved release heat, increasing the kinetic energy of the molecules in the surrounding environment. This released energy can then be used to generate electricity in power plants or to power vehicles.
Applications of Thermal Energy in Everyday Life
Beyond large-scale energy production, thermal energy plays a crucial role in countless everyday applications:
- Cooking: Cooking relies on transferring thermal energy to food, increasing the kinetic energy of its molecules and altering its properties. Whether you're using a gas stove, an electric oven, or a microwave, the basic principle remains the same: raising the temperature to cook the food.
- Heating and Cooling: Heating and cooling systems in our homes and buildings manage thermal energy transfer. Heating systems add thermal energy to the environment, increasing the kinetic energy of the air molecules, while cooling systems remove thermal energy, reducing the air's kinetic energy.
- Industrial Processes: Many industrial processes rely heavily on thermal energy. From smelting metals to manufacturing plastics, heat is essential to drive chemical reactions and shape materials.
- Transportation: Internal combustion engines in vehicles rely on the controlled burning of fuels to generate thermal energy, which is then converted into mechanical work to power the vehicle.
The Science Behind Thermal Energy Transfer
Understanding how thermal energy transfers is essential for many applications. Three primary mechanisms govern this transfer:
1. Conduction: Direct Transfer of Energy
Conduction is the transfer of thermal energy through direct contact between particles. When a hot object touches a cold one, the more energetic particles in the hot object collide with the less energetic particles in the cold object, transferring energy. Metals are excellent conductors due to their free-moving electrons, allowing for efficient energy transfer.
2. Convection: Transfer Through Fluid Movement
Convection occurs in fluids (liquids and gases) where thermal energy is transferred through the movement of heated particles. Warmer, less dense fluid rises, while cooler, denser fluid sinks, creating a cycle of heat transfer. This is how heating systems in buildings often work, circulating warm air throughout the space.
3. Radiation: Energy Transfer Through Electromagnetic Waves
Radiation is the transfer of thermal energy through electromagnetic waves. Unlike conduction and convection, radiation doesn't require a medium. The sun's energy reaches Earth through radiation. Infrared radiation is a primary form of thermal radiation.
Measuring Thermal Energy
The measurement of thermal energy is critical in various applications. While temperature gauges measure the average kinetic energy, determining the total thermal energy requires considering the mass and specific heat capacity of a substance.
- Specific Heat Capacity: This property represents the amount of energy required to raise the temperature of one unit of mass of a substance by one degree. Different substances have different specific heat capacities. Water, for example, has a high specific heat capacity, meaning it can absorb a significant amount of energy without a large temperature change.
Challenges and Future Directions
While harnessing thermal energy offers significant benefits, challenges remain:
- Efficiency: Converting thermal energy into other forms of energy, like electricity, isn't always highly efficient. Improving the efficiency of energy conversion processes is crucial for maximizing the benefits of thermal energy sources.
- Environmental Impact: The extraction and utilization of fossil fuels have significant environmental consequences. Developing cleaner and more sustainable methods of energy production, such as geothermal and solar energy, are essential for mitigating these impacts.
- Storage: Storing thermal energy effectively is challenging. Developing advanced energy storage solutions is vital for ensuring consistent energy supply from intermittent sources like solar and wind power.
Research into new materials, advanced energy conversion technologies, and improved energy storage solutions is ongoing. These advancements promise to unlock the full potential of thermal energy, paving the way for a more sustainable and energy-efficient future.
Conclusion
The movement of particles in a substance is a fundamental source of energy. Understanding this principle allows us to harness thermal energy for numerous applications, from generating electricity to heating our homes. However, addressing challenges related to efficiency, environmental impact, and storage remains crucial to fully realizing the potential of this ubiquitous energy source. Continued research and innovation promise to further refine our ability to tap into this abundant and readily available form of energy.
Latest Posts
Latest Posts
-
Group 1a On The Periodic Table
Mar 19, 2025
-
Periodic Table Of Elements Solid Liquid Gas
Mar 19, 2025
-
Nursing Documentation For Im Injection Example
Mar 19, 2025
-
How Is A Clumped Population Distribution Beneficial For Prey Animals
Mar 19, 2025
-
What Is A Subdivision In Music
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Energy Produced From The Movement Of Particles In A Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
