Equal To 1/12 The Mass Of A Carbon-12 Atom
Muz Play
May 11, 2025 · 6 min read
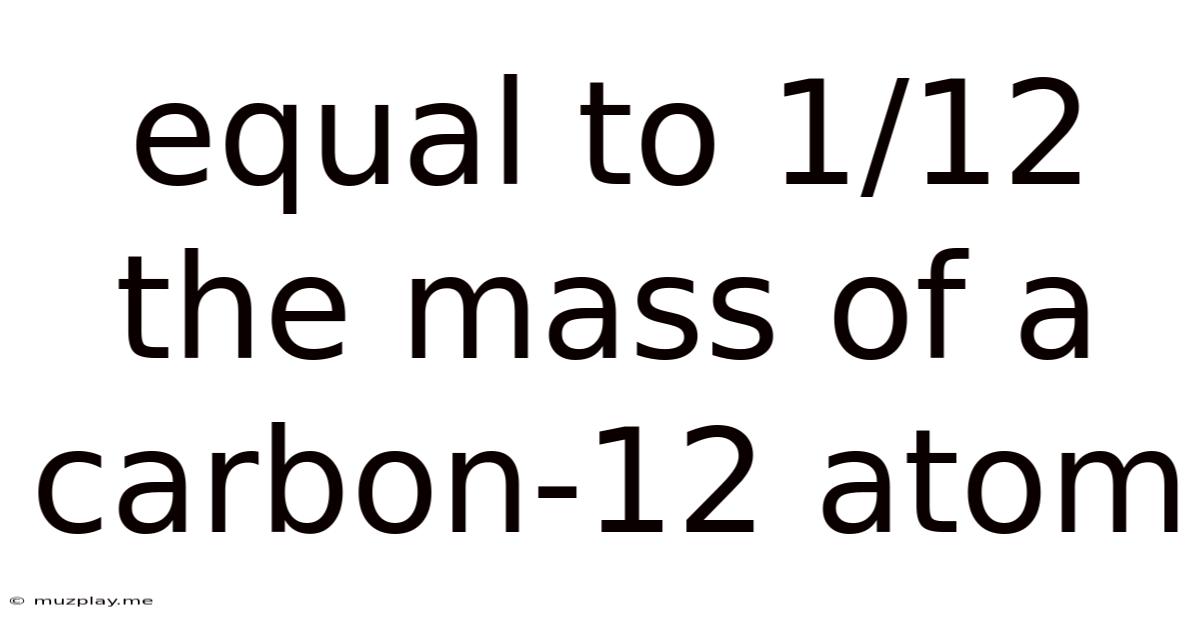
Table of Contents
Unveiling the Amu: Defining the Atomic Mass Unit (amu) and its Significance
The seemingly simple statement "equal to 1/12 the mass of a carbon-12 atom" holds profound implications in the world of chemistry and physics. This definition describes the atomic mass unit (amu), also known as the dalton (Da), a fundamental unit for expressing the mass of atoms and molecules. Understanding the amu is crucial for grasping the quantitative aspects of chemical reactions, isotopic abundances, and molecular structures. This comprehensive exploration delves into the history, definition, applications, and significance of this vital unit.
The Genesis of the Atomic Mass Unit: A Historical Perspective
Before the precise definition based on carbon-12, the determination of atomic masses was a complex and evolving process. Early chemists, like John Dalton, attempted to establish relative atomic weights based on experimental observations and chemical reactions. However, the lack of standardized reference points led to inconsistencies and inaccuracies. Different scales emerged, with hydrogen initially serving as the reference standard. Later, oxygen (both O-16 and a mixture of isotopes) was considered, highlighting the need for a more robust and universally accepted approach.
The adoption of carbon-12 as the foundation for the atomic mass unit marked a significant turning point. The International Union of Pure and Applied Chemistry (IUPAC) and the International Union of Pure and Applied Physics (IUPAP) collaborated to establish this standard in 1961. The choice of carbon-12 was driven by several factors: its abundance, ease of handling, and the precision with which its mass could be determined using mass spectrometry.
Why Carbon-12? The Advantages of a Stable Isotope
Selecting a specific isotope, rather than a naturally occurring mixture, eliminated the ambiguity arising from isotopic variations. Carbon-12, a stable and abundant isotope, offered the required precision and repeatability crucial for establishing a universally accepted standard. The use of a single isotope ensures that the amu remains consistent regardless of the source of the carbon-12 atom. This standardization revolutionized the accuracy and consistency of atomic mass measurements.
Defining the Amu: A Foundation for Chemical Calculations
The amu is defined as one-twelfth (1/12) the mass of a single, unbound atom of carbon-12 in its electronic ground state. This meticulously defined value forms the bedrock for expressing the mass of other atoms and molecules. It's important to note that the amu refers to the mass of an atom, not its weight. Mass is an intrinsic property of matter, while weight is influenced by gravitational forces.
The Significance of "Unbound" and "Ground State"
The terms "unbound" and "ground state" in the definition are crucial for ensuring precision and avoiding ambiguity. "Unbound" clarifies that the mass measured excludes any binding energy associated with molecules or crystals. "Ground state" specifies that the atom is in its lowest energy level, further minimizing potential variations due to excited states.
Applications of the Atomic Mass Unit: A Multifaceted Tool
The amu finds widespread applications across numerous scientific disciplines:
1. Determining Atomic and Molecular Masses:
The amu allows scientists to easily calculate the relative atomic mass of elements and the molecular mass of compounds. By knowing the atomic masses of constituent atoms, the molecular mass can be determined through simple addition, considering the number of atoms of each element in the molecule. This information is pivotal in stoichiometric calculations, determining the quantities of reactants and products in chemical reactions.
2. Isotope Abundance Calculations:
Natural elements exist as mixtures of isotopes, each with a slightly different mass. The amu, along with isotopic abundance data, allows for the calculation of the average atomic mass of an element found in nature. This average atomic mass is the value reported in the periodic table and is essential for performing real-world chemical calculations.
3. Mass Spectrometry:
Mass spectrometry is a powerful analytical technique used to identify and quantify molecules based on their mass-to-charge ratio. The amu provides a crucial unit for expressing the mass of ions detected by mass spectrometers, enabling the precise determination of molecular weights and isotopic compositions.
4. Nuclear Physics and Radioactivity:
In nuclear physics, the amu is essential for understanding nuclear reactions, decay processes, and mass-energy equivalence as described by Einstein's famous equation, E=mc². The precise mass differences before and after nuclear reactions can be expressed using the amu, revealing the energy released or absorbed during these transformations.
5. Biochemistry and Molecular Biology:
The amu is critical in biochemistry and molecular biology for determining the mass of biomolecules like proteins, DNA, and RNA. This information is essential for studying protein structures, understanding gene expression, and characterizing biological macromolecules.
6. Material Science and Nanotechnology:
In material science and nanotechnology, the amu plays a crucial role in characterizing materials at the atomic and molecular level. The precise mass measurements enable the development of novel materials with specific properties, contributing to advancements in diverse fields.
Beyond the Amu: Exploring Related Concepts
Several related concepts build upon the foundation of the atomic mass unit:
1. Mole and Avogadro's Number:
The mole (mol) is a fundamental unit in chemistry representing a specific number of entities (atoms, molecules, ions, etc.). Avogadro's number (approximately 6.022 x 10²³) specifies the number of entities in one mole. The combination of the amu and Avogadro's number allows for the conversion between the mass of a substance and the number of moles, facilitating stoichiometric calculations.
2. Relative Atomic Mass and Relative Molecular Mass:
Relative atomic mass (Ar) and relative molecular mass (Mr) are dimensionless quantities representing the average mass of an atom or molecule relative to 1/12 the mass of a carbon-12 atom. These values are commonly used in stoichiometry and other chemical calculations and are readily derived using the amu.
3. Unified Atomic Mass Unit (u):
While often used interchangeably with amu, the unified atomic mass unit (u) is sometimes preferred to avoid confusion with other uses of "amu." However, both units represent the same fundamental quantity.
The Enduring Importance of the Atomic Mass Unit
The definition "equal to 1/12 the mass of a carbon-12 atom" represents a remarkable achievement in scientific standardization. The amu provides a consistent and precise unit for expressing atomic and molecular masses, underpinning numerous scientific calculations and analyses across diverse fields. From basic chemistry to advanced physics and biochemistry, the amu remains a cornerstone of modern science, facilitating our understanding of the fundamental building blocks of matter. Its continued use ensures accuracy and consistency in scientific research and applications, a testament to the importance of precise and universally accepted standards. The amu's enduring significance is a clear reflection of its role in building a coherent and accurate understanding of the physical world around us. Its future relevance in fields like nanotechnology and advanced materials science ensures its ongoing prominence in scientific endeavor.
Latest Posts
Latest Posts
-
What Important Idea From Thomas Malthus Inspired Darwin
May 12, 2025
-
The Opportunity Cost Of Holding Money Balances Increases When
May 12, 2025
-
Side By Side Stem And Leaf Plot
May 12, 2025
-
Convert The Polar Equation To A Rectangular Equation
May 12, 2025
-
What Type Of Reaction Is Photosynthesis Anabolic Or Catabolic
May 12, 2025
Related Post
Thank you for visiting our website which covers about Equal To 1/12 The Mass Of A Carbon-12 Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.