Equation For Ionization Of Acetic Acid
Muz Play
Mar 15, 2025 · 5 min read
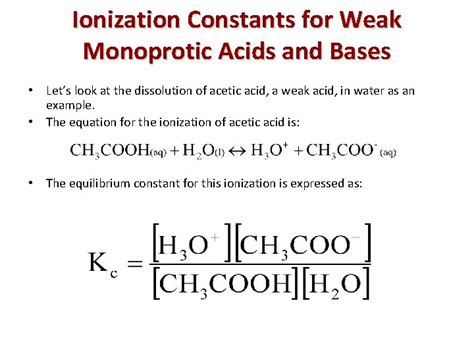
Table of Contents
The Equation for the Ionization of Acetic Acid: A Deep Dive
Acetic acid, also known as ethanoic acid, is a weak organic acid with the chemical formula CH₃COOH. Understanding its ionization equation is crucial in various fields, including chemistry, biochemistry, and environmental science. This article will provide a comprehensive exploration of the ionization equation for acetic acid, delving into its equilibrium constant, factors influencing ionization, and practical applications.
Understanding the Ionization Process
Acetic acid, unlike strong acids like hydrochloric acid (HCl), does not fully dissociate in aqueous solutions. Instead, it undergoes partial ionization, meaning only a fraction of its molecules donate a proton (H⁺) to water molecules. This process establishes an equilibrium between the undissociated acetic acid molecules and its constituent ions.
The Equilibrium Reaction
The ionization of acetic acid can be represented by the following equilibrium reaction:
CH₃COOH(aq) + H₂O(l) ⇌ CH₃COO⁻(aq) + H₃O⁺(aq)
This equation shows that acetic acid (CH₃COOH) reacts with water (H₂O) to produce acetate ions (CH₃COO⁻) and hydronium ions (H₃O⁺). The double arrow (⇌) signifies that the reaction proceeds in both forward and reverse directions simultaneously, reaching a state of dynamic equilibrium.
The Acid Dissociation Constant (Ka)
The equilibrium constant for the ionization of a weak acid like acetic acid is called the acid dissociation constant, denoted as K<sub>a</sub>. For acetic acid, K<sub>a</sub> is defined as:
K<sub>a</sub> = [CH₃COO⁻][H₃O⁺] / [CH₃COOH]
where:
- [CH₃COO⁻] represents the equilibrium concentration of acetate ions.
- [H₃O⁺] represents the equilibrium concentration of hydronium ions.
- [CH₃COOH] represents the equilibrium concentration of undissociated acetic acid.
The value of K<sub>a</sub> for acetic acid is approximately 1.8 x 10⁻⁵ at 25°C. This small value indicates that acetic acid is a weak acid, meaning it only partially ionizes in solution. The lower the K<sub>a</sub> value, the weaker the acid.
Factors Affecting the Ionization of Acetic Acid
Several factors influence the extent to which acetic acid ionizes in solution. These include:
1. Concentration of Acetic Acid
The concentration of acetic acid directly affects its degree of ionization. A more dilute solution will exhibit a higher percentage ionization compared to a concentrated solution. This is a consequence of Le Chatelier's principle; diluting the solution shifts the equilibrium towards the right, favoring the formation of ions.
2. Temperature
Temperature also plays a significant role. Increasing the temperature generally increases the degree of ionization. Higher temperatures provide more energy to overcome the energy barrier required for the dissociation process.
3. Presence of Common Ions
The presence of common ions, such as acetate ions (from sodium acetate, for example), suppresses the ionization of acetic acid. This phenomenon, known as the common ion effect, is another consequence of Le Chatelier's principle. The addition of acetate ions shifts the equilibrium to the left, reducing the concentration of H₃O⁺ ions and thus lowering the pH.
4. Solvent Effects
The solvent used can also impact ionization. The dielectric constant of the solvent influences the electrostatic interactions between the ions, affecting the extent of ionization. In solvents with higher dielectric constants, ionization is generally favored.
Applications of the Ionization Equation
The ionization equation for acetic acid and its associated equilibrium constant have numerous applications in various fields:
1. Buffer Solutions
Acetic acid, in conjunction with its conjugate base (acetate ion), forms a crucial component of buffer solutions. Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. The Henderson-Hasselbalch equation, derived from the K<sub>a</sub> expression, is used to calculate the pH of buffer solutions containing acetic acid and acetate ions.
2. Titration Calculations
The ionization equation is essential in acid-base titrations involving acetic acid. Understanding the equilibrium allows accurate calculation of the pH at different points during the titration, enabling determination of the equivalence point.
3. Biochemical Processes
Acetic acid plays a vital role in various biochemical processes. Its ionization is relevant in understanding the behavior of acetic acid in biological systems, influencing enzymatic reactions and maintaining cellular pH.
4. Environmental Chemistry
Acetic acid is found in the environment, contributing to the acidity of rainwater and soils. Its ionization is important in assessing the environmental impact of acid rain and understanding the fate and transport of acetic acid in various ecosystems.
5. Industrial Applications
Acetic acid is used extensively in industries like food processing, pharmaceuticals, and textiles. Understanding its ionization behavior is crucial for optimizing processes and ensuring product quality.
Beyond the Simple Equation: A More Complex Reality
While the simple ionization equation provides a good foundational understanding, a more nuanced approach is often necessary. Factors such as activity coefficients and ionic strength, which are not explicitly accounted for in the simple K<sub>a</sub> expression, can significantly influence the actual degree of ionization in real-world solutions.
Activity Coefficients
In concentrated solutions, the interaction between ions becomes significant, leading to deviations from ideal behavior. Activity coefficients correct for these non-ideal interactions, representing the effective concentration of ions in solution. The thermodynamically rigorous expression for K<sub>a</sub> incorporates activity coefficients:
K<sub>a</sub> = (γ<sub>CH₃COO⁻</sub>[CH₃COO⁻])(γ<sub>H₃O⁺</sub>[H₃O⁺]) / (γ<sub>CH₃COOH</sub>[CH₃COOH])
where γ represents the activity coefficient for each species. Calculating activity coefficients often involves complex models like the Debye-Hückel theory.
Ionic Strength
Ionic strength, a measure of the total concentration of ions in a solution, significantly influences activity coefficients. Higher ionic strength typically leads to lower activity coefficients, reducing the effective concentration of ions and impacting the degree of ionization.
Conclusion
The ionization equation for acetic acid, CH₃COOH(aq) + H₂O(l) ⇌ CH₃COO⁻(aq) + H₃O⁺(aq), is fundamental to understanding its behavior in aqueous solutions. While the simple K<sub>a</sub> expression provides a valuable approximation, a more thorough understanding necessitates considering factors like concentration, temperature, common ion effects, solvent properties, activity coefficients, and ionic strength. The ionization of acetic acid has significant implications across various scientific and industrial fields, highlighting its importance in chemistry, biochemistry, and environmental science. A comprehensive understanding of this seemingly simple equation unlocks a deeper appreciation of its broader relevance in the natural world and human applications. Further research into the intricacies of activity coefficients and ionic strength will provide a more precise and accurate representation of acetic acid's ionization behavior in various conditions.
Latest Posts
Latest Posts
-
Adjusting Entries And Adjusted Trial Balance
May 09, 2025
-
What Is The Group Number For Sulfur
May 09, 2025
-
Which Stage Of Aerobic Respiration Requires An Input Of Oxygen
May 09, 2025
-
Five Carbon Sugar Found In Dna
May 09, 2025
-
The Oxygen Isotope With 8 Neutrons
May 09, 2025
Related Post
Thank you for visiting our website which covers about Equation For Ionization Of Acetic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.