Equilibrium Is Reached When What Occurs
Muz Play
Mar 30, 2025 · 6 min read
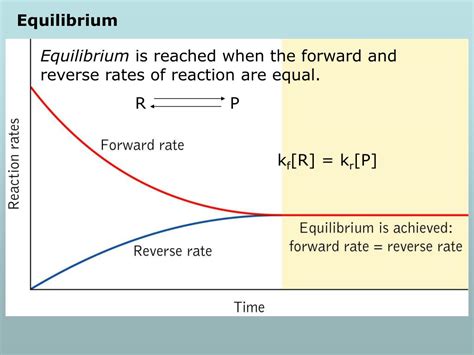
Table of Contents
Equilibrium: When the Rates of Forward and Reverse Reactions Become Equal
Equilibrium, a cornerstone concept in chemistry and physics, isn't a static state of nothingness. Instead, it represents a dynamic balance where opposing processes occur at equal rates. Understanding when equilibrium is reached requires grasping the interplay between forward and reverse reactions, and how this balance is affected by various factors. This article delves deep into the concept of equilibrium, exploring its implications across diverse scientific domains.
Understanding Chemical Equilibrium: A Dynamic Balance
Chemical equilibrium is achieved in a reversible reaction when the rate of the forward reaction (reactants forming products) equals the rate of the reverse reaction (products forming reactants). It's crucial to understand that this doesn't mean the concentrations of reactants and products are equal; rather, it signifies that the rates of the opposing reactions are identical. Imagine a busy highway with cars flowing in both directions. Equilibrium is like reaching a point where the number of cars going one way precisely matches the number going the other way, even if the total number of cars on each side of the highway differs.
The Concept of Reversible Reactions
The very foundation of equilibrium lies in the concept of reversible reactions. Unlike irreversible reactions that proceed in only one direction until completion, reversible reactions can proceed in both forward and backward directions simultaneously. A classic example is the synthesis of ammonia from nitrogen and hydrogen gases:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
The double arrow (⇌) indicates the reversibility of this reaction. At equilibrium, nitrogen and hydrogen continuously react to form ammonia, while simultaneously, ammonia decomposes back into nitrogen and hydrogen.
Factors Affecting Equilibrium: Le Chatelier's Principle
Henri Louis Le Chatelier's principle provides a powerful tool for predicting the effect of external changes on a system at equilibrium. This principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. These changes include:
-
Changes in Concentration: Increasing the concentration of a reactant will shift the equilibrium to favor the forward reaction, producing more products. Conversely, increasing the concentration of a product will shift the equilibrium towards the reverse reaction, producing more reactants.
-
Changes in Pressure: Changes in pressure primarily affect gaseous equilibrium systems. Increasing the pressure favors the side with fewer gas molecules, while decreasing the pressure favors the side with more gas molecules. For the ammonia synthesis reaction, increasing pressure favors the formation of ammonia (fewer gas molecules on the product side).
-
Changes in Temperature: Temperature changes affect the equilibrium constant (K). For exothermic reactions (those that release heat), increasing the temperature shifts the equilibrium to the left (favoring reactants), while decreasing the temperature favors the forward reaction. For endothermic reactions (those that absorb heat), the opposite is true.
Equilibrium Constant (K): A Quantitative Measure of Equilibrium
The equilibrium constant, K, provides a quantitative measure of the relative amounts of reactants and products at equilibrium. For the general reversible reaction:
aA + bB ⇌ cC + dD
The equilibrium constant is expressed as:
K = ([C]<sup>c</sup>[D]<sup>d</sup>) / ([A]<sup>a</sup>[B]<sup>b</sup>)
where [A], [B], [C], and [D] represent the equilibrium concentrations of the respective species, and a, b, c, and d are their stoichiometric coefficients. A large K value indicates that the equilibrium favors the formation of products, while a small K value signifies that the equilibrium favors the reactants.
Equilibrium in Different Contexts
Equilibrium isn't confined to chemical reactions; it's a fundamental principle across various scientific disciplines:
Phase Equilibrium
Phase equilibrium refers to the coexistence of multiple phases of a substance at a specific temperature and pressure. For example, the equilibrium between ice, liquid water, and water vapor at 0°C and 1 atm is a classic illustration of phase equilibrium.
Solution Equilibrium
Solution equilibrium involves the dissolution of a solute in a solvent. The equilibrium constant in this case is the solubility product (Ksp) for slightly soluble ionic compounds. Ksp reflects the extent to which a compound dissolves in a given solvent.
Thermal Equilibrium
Thermal equilibrium refers to the state where two or more objects or systems in thermal contact have reached the same temperature and no net heat transfer occurs between them. This is a fundamental principle in thermodynamics.
Mechanical Equilibrium
Mechanical equilibrium is achieved when there's no net force acting on a system. All forces are balanced, resulting in no acceleration. This is relevant in mechanics and structural engineering.
Applications of Equilibrium Principles
The understanding and application of equilibrium principles are crucial across numerous fields:
-
Chemical Engineering: Equilibrium calculations are vital in designing and optimizing chemical reactors, ensuring efficient product formation.
-
Environmental Science: Equilibrium concepts are used to model and predict pollutant behavior in the environment, assessing their impact on ecosystems.
-
Biochemistry: Enzyme kinetics and metabolic pathways involve intricate equilibrium processes, essential for understanding biological systems.
-
Materials Science: Equilibrium diagrams (phase diagrams) are instrumental in predicting the properties and behavior of materials under various conditions.
Equilibrium and Kinetics: A Subtle Difference
While both equilibrium and kinetics deal with chemical reactions, they focus on different aspects. Kinetics studies the rate of a reaction, exploring factors that influence how quickly reactants are converted into products. Equilibrium, on the other hand, focuses on the relative amounts of reactants and products at a state of dynamic balance. It's important to remember that a fast reaction rate doesn't necessarily mean a large equilibrium constant. A reaction could proceed rapidly but still favor reactants at equilibrium.
Disturbances to Equilibrium and the Shift Towards a New Equilibrium
As mentioned earlier, Le Chatelier's principle elegantly predicts how a system at equilibrium responds to external disturbances. However, it's crucial to understand that these shifts are temporary. The system will eventually reach a new equilibrium state, where the rates of forward and reverse reactions are again equal, albeit with different concentrations of reactants and products compared to the initial equilibrium. This new equilibrium reflects the system’s adaptation to the imposed change. The system doesn't simply remain in a state of imbalance indefinitely.
Calculating Equilibrium Concentrations
Determining equilibrium concentrations is often necessary in various applications. This often involves solving simultaneous equations that represent the equilibrium expressions and the stoichiometry of the reaction. Techniques like ICE tables (Initial, Change, Equilibrium) provide a structured approach to systematically track changes in concentrations during the approach to equilibrium.
Advanced Topics in Equilibrium
More advanced treatments of equilibrium encompass concepts such as:
- Activity Coefficients: These factors correct for non-ideal behavior in solutions, improving the accuracy of equilibrium calculations.
- Temperature Dependence of Equilibrium Constants: The van't Hoff equation describes how the equilibrium constant changes with temperature.
- Coupled Equilibria: Many real-world systems involve multiple coupled equilibria, requiring more complex analysis.
Conclusion: The Dynamic Nature of Equilibrium
Equilibrium isn't a static endpoint but rather a dynamic interplay of opposing processes. Understanding when equilibrium is reached requires considering the rates of forward and reverse reactions and how external factors can influence this balance. This principle permeates various scientific fields, providing a fundamental framework for understanding diverse systems and phenomena. From chemical reactions to phase transitions, the principles of equilibrium offer a powerful lens through which we can analyze and predict the behavior of the physical world. The dynamic balance of equilibrium underscores its importance as a cornerstone concept in science and engineering.
Latest Posts
Latest Posts
-
Journal Entry To Issue Common Stock
Apr 01, 2025
-
Real Life Examples Of Linear Equations In Two Variable
Apr 01, 2025
-
What Does An Mean In Arithmetic Sequences
Apr 01, 2025
-
How To Find Pmf From Cdf
Apr 01, 2025
-
Area By Integration Problems With Solutions
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Equilibrium Is Reached When What Occurs . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
