Example Of Solid Dissolved In Liquid
Muz Play
Apr 01, 2025 · 6 min read
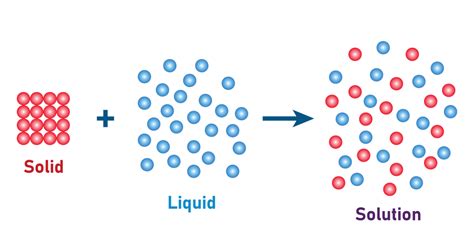
Table of Contents
Examples of Solids Dissolved in Liquids: A Deep Dive into Solutions
Solutions are ubiquitous in our daily lives, from the saltwater in the ocean to the electrolytes in our bodies. Understanding how solids dissolve in liquids is fundamental to chemistry and numerous applications across various fields. This comprehensive article explores the fascinating world of solid-in-liquid solutions, providing numerous examples, explanations of the underlying principles, and real-world applications.
What is a Solution?
Before delving into specific examples, let's define what constitutes a solution. A solution is a homogeneous mixture of two or more substances. In a solid-in-liquid solution, a solid solute is uniformly dispersed throughout a liquid solvent. The solute is the substance being dissolved (the solid), and the solvent is the substance doing the dissolving (the liquid). The resulting solution is a single phase, meaning it has a uniform composition throughout. This is in contrast to a suspension, where the solid particles remain visible and settle over time.
Key characteristics of a solution include:
- Homogeneity: The solute is evenly distributed throughout the solvent.
- Transparency: Solutions are typically transparent, although the color may vary depending on the solute and solvent.
- Particle size: The solute particles are at the atomic or molecular level, too small to be seen with the naked eye.
- Stability: Solutions are stable, meaning the solute does not settle out of the solvent over time.
Factors Affecting Solubility
The ability of a solid to dissolve in a liquid depends on several factors:
-
Nature of the solute and solvent: "Like dissolves like" is a crucial principle. Polar solvents (like water) tend to dissolve polar solutes (like sugar), while nonpolar solvents (like oil) dissolve nonpolar solutes (like fats). This is due to the intermolecular forces between the solute and solvent molecules. Stronger interactions lead to higher solubility.
-
Temperature: Increasing the temperature usually increases the solubility of solids in liquids. The added kinetic energy allows the solvent molecules to overcome the intermolecular forces holding the solid together.
-
Pressure: Pressure generally has a minimal effect on the solubility of solids in liquids. The effect is more pronounced for gases dissolved in liquids.
-
Particle size: Smaller solute particles dissolve faster than larger ones because of increased surface area.
Examples of Solids Dissolved in Liquids
Now, let's explore numerous examples of solids dissolved in liquids, categorized for clarity:
Everyday Examples:
-
Sugar in water: This is perhaps the most common example. Sugar (sucrose), a polar molecule, readily dissolves in water, a polar solvent, forming a homogeneous solution. The hydroxyl groups (-OH) on the sugar molecule form hydrogen bonds with water molecules.
-
Salt (NaCl) in water: Table salt, sodium chloride, is an ionic compound that readily dissolves in water. The polar water molecules surround the sodium and chloride ions, weakening the ionic bonds and allowing them to separate and disperse throughout the solution. This process is called hydration.
-
Coffee or tea: These beverages contain numerous dissolved solids, including caffeine, tannins, and various sugars and minerals. The hot water acts as a solvent, extracting these compounds from the coffee grounds or tea leaves.
-
Soft drinks: Carbonated beverages contain dissolved sugars, flavorings, and carbon dioxide (although CO2 is a gas, it is often dissolved under pressure).
-
Ocean water: Seawater is a complex solution containing various dissolved salts, minerals, and gases. Sodium chloride is the most abundant salt, but many other ions like magnesium, calcium, and potassium are also present.
Industrial and Scientific Examples:
-
Sodium hydroxide (NaOH) in water: This forms a highly alkaline solution used in many industrial processes, including cleaning, soap making, and chemical synthesis.
-
Potassium permanganate (KMnO4) in water: This produces a deep purple solution used as an oxidizing agent in various chemical reactions and as a disinfectant.
-
Copper sulfate (CuSO4) in water: This creates a blue solution used in agriculture as a fungicide and algaecide, and in electroplating.
-
Silver nitrate (AgNO3) in water: This forms a colorless solution used in photography and as a reagent in chemical analysis.
-
Various metal salts in water: Many metal salts, such as zinc sulfate, iron chloride, and nickel nitrate, dissolve in water to create solutions used in various industrial processes, such as electroplating, catalysis, and pigment production.
Biological Examples:
-
Glucose in blood: Glucose, a simple sugar, is dissolved in the bloodstream and transported to cells for energy production.
-
Electrolytes in bodily fluids: Essential ions like sodium, potassium, calcium, and chloride are dissolved in bodily fluids and play vital roles in nerve impulse transmission, muscle contraction, and maintaining osmotic balance.
-
Minerals in plant sap: Plants absorb minerals from the soil, which are then dissolved in the plant sap and transported to different parts of the plant.
Applications of Solid-in-Liquid Solutions
Solid-in-liquid solutions are essential in a vast array of applications:
-
Medicine: Many drugs are administered in solution form for better absorption and bioavailability. Intravenous fluids contain dissolved salts and sugars to maintain electrolyte balance and provide hydration.
-
Agriculture: Fertilizers often contain dissolved nutrients that plants can easily absorb.
-
Food industry: Many food products are solutions, including syrups, sauces, and beverages.
-
Chemical industry: Numerous industrial processes rely on chemical reactions that occur in solution.
-
Environmental science: The analysis of water quality involves determining the concentrations of dissolved solids and pollutants.
-
Material science: Solutions are used to create materials with specific properties, such as coatings, composites, and thin films.
Understanding the Dissolution Process at a Molecular Level
The dissolution of a solid in a liquid is a dynamic process involving several steps:
-
Solvation: The solvent molecules surround the solute particles, weakening the attractive forces between the solute particles.
-
Dissociation: For ionic compounds, the solvent molecules can completely separate the ions, leading to dissociation. For molecular compounds, the molecules remain intact but are dispersed throughout the solvent.
-
Diffusion: The dissolved solute particles spread throughout the solvent, driven by entropy (the tendency towards randomness).
The rate of dissolution depends on factors like the surface area of the solid, the temperature, and the agitation of the solution. Stirring or shaking the solution increases the rate of dissolution by increasing the contact between the solvent and the solute.
Unsaturated, Saturated, and Supersaturated Solutions
The concentration of a solution plays a significant role in its properties. We can categorize solutions based on their concentration:
-
Unsaturated solution: A solution where the solvent can still dissolve more solute at a given temperature and pressure.
-
Saturated solution: A solution where the solvent has dissolved the maximum amount of solute at a given temperature and pressure. Any additional solute will remain undissolved.
-
Supersaturated solution: A solution that contains more solute than it can normally dissolve at a given temperature and pressure. These solutions are unstable and can easily precipitate the excess solute.
Conclusion
Solid-in-liquid solutions are fundamental to chemistry and numerous applications in our daily lives and various industries. Understanding the factors that affect solubility, the different types of solutions, and their diverse applications is crucial for scientists, engineers, and anyone interested in the fascinating world of chemistry. This article provides a broad overview, highlighting the importance of this ubiquitous phenomenon, its practical applications, and the intricate molecular processes involved. From the simple act of dissolving sugar in water to the complex chemical processes in industrial settings and biological systems, the dissolution of solids in liquids is a vital aspect of our world.
Latest Posts
Latest Posts
-
Is Sugar A Pure Substance Or A Mixture
Apr 02, 2025
-
Orange Juice Is Acid Or Base
Apr 02, 2025
-
When Does Segregation Occur In Meiosis
Apr 02, 2025
-
A Worm Is Living Inside A Cow
Apr 02, 2025
-
What Plane Divides The Body Into Front And Back Portions
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Example Of Solid Dissolved In Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
