Final Acceptor Of Electrons In The Electron Transport Chain
Muz Play
Mar 22, 2025 · 6 min read
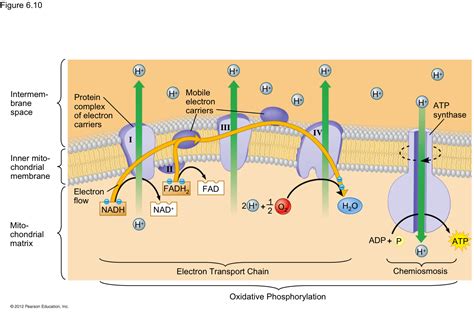
Table of Contents
The Final Electron Acceptor in the Electron Transport Chain: Oxygen and its Crucial Role in Cellular Respiration
The electron transport chain (ETC), a cornerstone of cellular respiration, is a series of protein complexes embedded within the inner mitochondrial membrane (in eukaryotes) or the plasma membrane (in prokaryotes). Its primary function is to harness the energy stored in high-energy electrons, ultimately generating a proton gradient that drives ATP synthesis – the cell's primary energy currency. While the entire chain is crucial, the final electron acceptor plays a pivotal role, determining the efficiency and overall outcome of the process. This article delves deep into the nature of this final acceptor, its significance, and the consequences of its absence or alteration.
Understanding the Electron Transport Chain
Before we focus on the final electron acceptor, it's crucial to understand the broader context of the ETC. The chain operates through a series of redox reactions, where electrons are passed from one molecule to another, each with a progressively higher electron affinity. This stepwise transfer releases energy, which is cleverly utilized to pump protons (H+) across the inner mitochondrial membrane, creating an electrochemical gradient. This gradient, encompassing both a chemical concentration difference and an electrical potential, stores potential energy.
This potential energy is then harnessed by ATP synthase, a remarkable molecular machine that uses the proton flow back across the membrane to synthesize ATP from ADP and inorganic phosphate (Pi). This process, known as chemiosmosis, is the main mechanism of ATP production in aerobic respiration.
The ETC consists of four major protein complexes (Complexes I-IV), along with two mobile electron carriers: ubiquinone (CoQ) and cytochrome c. Each complex facilitates electron transfer, contributing to the proton gradient generation. Let's briefly summarize the electron flow:
- Complex I (NADH dehydrogenase): Receives electrons from NADH, a high-energy electron carrier produced during glycolysis and the citric acid cycle.
- Complex II (Succinate dehydrogenase): Receives electrons from FADH2, another high-energy electron carrier produced during the citric acid cycle.
- Ubiquinone (CoQ): A lipid-soluble molecule that acts as a mobile electron carrier, transferring electrons between Complex I/II and Complex III.
- Complex III (Cytochrome bc1 complex): Receives electrons from ubiquinone and further contributes to proton pumping.
- Cytochrome c: A water-soluble protein that acts as a mobile electron carrier, transferring electrons between Complex III and Complex IV.
- Complex IV (Cytochrome c oxidase): The final complex in the chain, receiving electrons from cytochrome c.
Oxygen: The Ultimate Electron Acceptor
Now, let's address the critical question: what receives the electrons at the end of this intricate chain? In aerobic respiration, the final electron acceptor is molecular oxygen (O2). This is a crucial point, as it defines the process as aerobic. The reduction of oxygen to water is the crucial final step, completing the electron transport chain and enabling the entire process to continue.
The reaction at Complex IV is:
4e- + 4H+ + O2 → 2H2O
This reaction is catalyzed by cytochrome c oxidase, the enzyme that forms Complex IV. The reduction of oxygen to water is essential because it prevents the buildup of electrons within the ETC. Without a final electron acceptor, the electron transport chain would become "backed up," halting ATP synthesis and ultimately leading to cellular dysfunction.
The Significance of Oxygen's Role
Oxygen's role is not merely the final step; it's the linchpin of the entire system. Its high electronegativity allows it to readily accept electrons, driving the entire process forward. The energy released during oxygen reduction is essential for the proton pumping action of the complexes, making it a key player in generating the proton gradient responsible for ATP production.
Without oxygen, the ETC would cease to function. This is why oxygen is essential for aerobic respiration, the primary metabolic pathway for ATP production in most organisms. The absence of oxygen forces the cell to switch to anaerobic respiration or fermentation, processes that produce significantly less ATP.
Consequences of Oxygen Absence or Alteration
The consequences of oxygen deprivation (hypoxia) or a reduction in oxygen availability (hypoxemia) are profound and far-reaching:
- Reduced ATP Production: The most immediate consequence is a drastic decrease in ATP production. Cells are forced to rely on less efficient anaerobic pathways, leading to energy deficiency.
- Metabolic Shift: Cells shift their metabolism from aerobic respiration to anaerobic respiration (e.g., lactic acid fermentation in muscle cells). This shift results in the production of metabolic byproducts such as lactate, which can lead to acidosis.
- Cellular Damage: The lack of ATP impairs various cellular processes, including protein synthesis, ion transport, and DNA replication and repair. This can lead to cellular damage and eventually cell death.
- Organ Dysfunction: Widespread hypoxia or hypoxemia can lead to organ dysfunction, as vital organs like the heart, brain, and kidneys require a continuous supply of ATP.
- Disease States: Chronic hypoxia is associated with several diseases, including cardiovascular disease, stroke, and chronic obstructive pulmonary disease (COPD).
Moreover, alterations in the ETC itself, including mutations affecting the complexes or the final electron acceptor binding site, can also lead to severe health consequences. These mutations can disrupt the electron flow, reduce ATP production, and potentially generate harmful reactive oxygen species (ROS).
Alternative Electron Acceptors in Anaerobic Respiration
While oxygen is the most efficient and common final electron acceptor, some organisms have evolved to use alternative electron acceptors in anaerobic conditions. These organisms, often found in oxygen-depleted environments, utilize different terminal oxidases, enzymes that catalyze the reduction of alternative acceptors. Examples include:
- Nitrate (NO3-): Used by denitrifying bacteria, reducing nitrate to nitrite (NO2-), nitric oxide (NO), nitrous oxide (N2O), and finally nitrogen gas (N2).
- Sulfate (SO42-): Used by sulfate-reducing bacteria, reducing sulfate to hydrogen sulfide (H2S).
- Fumarate: Used by certain bacteria, reducing fumarate to succinate.
- Carbon Dioxide (CO2): Used by methanogenic archaea, reducing carbon dioxide to methane (CH4).
These alternative electron acceptors are less efficient than oxygen, resulting in lower ATP yields. However, they allow these organisms to survive and thrive in environments lacking molecular oxygen.
The Importance of Studying the Final Electron Acceptor
Understanding the final electron acceptor and its role in the ETC is crucial for several reasons:
- Disease Research: Research into ETC dysfunction is vital for understanding and treating various diseases, including mitochondrial disorders, cardiovascular diseases, and cancer.
- Drug Development: Targeting the ETC components or influencing the final electron acceptor can be a valuable strategy for developing new drugs.
- Environmental Microbiology: Studying the use of alternative electron acceptors is crucial for understanding microbial ecology and biogeochemical cycles in various environments.
- Biotechnology: Harnessing the power of the ETC and manipulating the final electron acceptor can have applications in biotechnology, such as biofuel production.
Conclusion: Oxygen's Indispensable Role
In conclusion, oxygen's role as the final electron acceptor in the electron transport chain is not merely a final step but a vital element that underpins the efficiency and effectiveness of cellular respiration. Its high electronegativity ensures a continuous flow of electrons, allowing for maximal ATP production. The consequences of oxygen absence or alterations in the ETC are far-reaching, impacting cellular function, organ systems, and ultimately leading to various pathological conditions. Continued research into the intricacies of the electron transport chain and the role of the final electron acceptor remains crucial for advancing our understanding of cellular metabolism, disease mechanisms, and developing innovative therapeutic strategies. The study of alternative electron acceptors also expands our knowledge of microbial diversity and ecological processes. The intricate balance maintained by the ETC, ultimately dependent on the final electron acceptor, highlights the remarkable elegance and efficiency of cellular energy production.
Latest Posts
Latest Posts
-
How To Prove Function Is Onto
Mar 24, 2025
-
Pedigrees Practice Human Genetic Disorders
Mar 24, 2025
-
What Are The Characteristics Of Metals
Mar 24, 2025
-
A Bundle Of Axons In The Cns Is Called
Mar 24, 2025
-
Where Do The Light Independent Reactions Occur
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Final Acceptor Of Electrons In The Electron Transport Chain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
