First Order Versus Zero Order Kinetics
Muz Play
Mar 27, 2025 · 6 min read
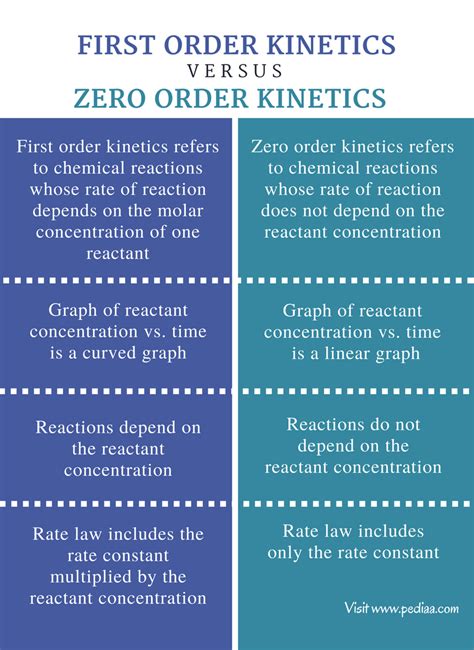
Table of Contents
First-Order vs. Zero-Order Kinetics: A Deep Dive into Pharmacokinetics
Understanding drug kinetics is crucial in pharmacology and pharmaceutical development. This article delves into the fundamental differences between first-order and zero-order kinetics, exploring their implications for drug absorption, distribution, metabolism, and excretion (ADME), dosage regimens, and therapeutic efficacy. We'll examine the mathematical models, provide real-world examples, and highlight the clinical significance of differentiating between these kinetic orders.
What are First-Order and Zero-Order Kinetics?
Drug kinetics describes how the concentration of a drug changes over time within the body. This change is governed by various processes, including absorption from the site of administration, distribution to different tissues, metabolism (biotransformation), and excretion (elimination). The rate at which these processes occur can be described by kinetic orders.
First-order kinetics is the most common type of drug elimination. In this scenario, the rate of elimination is directly proportional to the drug concentration. This means that the higher the drug concentration, the faster the drug is eliminated. A constant fraction of the drug is eliminated per unit of time.
Zero-order kinetics, on the other hand, is less common. In this case, the rate of elimination is constant, regardless of the drug concentration. This means a constant amount of drug is eliminated per unit of time. This usually occurs when the elimination process is saturated.
Mathematical Representation
The differences between first-order and zero-order kinetics are clearly illustrated by their mathematical equations:
First-Order Kinetics:
The rate of elimination is given by: dC/dt = -kC
Where:
dC/dtrepresents the change in drug concentration (C) over time (t).kis the elimination rate constant (a proportionality constant). This is a characteristic property of the drug and its metabolism.
Integrating this equation leads to the exponential decay equation:
C(t) = C₀e⁻ᵏᵗ
Where:
C(t)is the drug concentration at time t.C₀is the initial drug concentration.
Zero-Order Kinetics:
The rate of elimination is constant and given by:
dC/dt = -k₀
Where: k₀ is the zero-order rate constant (representing the constant rate of elimination).
Integrating this equation gives:
C(t) = C₀ - k₀t
This equation shows a linear decrease in drug concentration over time.
Factors Affecting Kinetic Order
Several factors can influence whether a drug exhibits first-order or zero-order kinetics:
-
Enzyme Saturation: Many drugs are metabolized by enzymes in the liver. At low drug concentrations, the enzymes are not saturated, and the rate of metabolism follows first-order kinetics. However, at high drug concentrations, the enzymes become saturated, leading to zero-order kinetics. This is because the rate-limiting step becomes the enzyme availability, not the drug concentration.
-
Drug Transport Mechanisms: Active transport systems involved in drug absorption and excretion can also become saturated at high drug concentrations, resulting in a shift from first-order to zero-order kinetics.
-
Drug Formulation: The formulation of a drug can affect its absorption rate. Slow-release formulations, for example, can lead to more consistent drug levels and maintain first-order kinetics over a longer period.
-
Patient Factors: Individual differences in liver function, kidney function, and genetic factors can affect drug metabolism and excretion rates, potentially altering the kinetic order.
Clinical Implications
The distinction between first-order and zero-order kinetics has significant clinical implications for:
-
Dosage Regimens: For drugs exhibiting first-order kinetics, the dosage regimen can be adjusted to achieve a desired steady-state concentration. A constant fraction is eliminated, meaning a constant dose will eventually lead to a predictable equilibrium. Zero-order kinetics, however, requires a more nuanced approach due to the constant amount of drug elimination.
-
Toxicity: Drugs exhibiting zero-order kinetics have a higher risk of toxicity at high doses because the rate of elimination is not proportional to the concentration. Even a small increase in dose can lead to a disproportionately large increase in plasma concentration, potentially exceeding the therapeutic window.
-
Drug Interactions: Drugs that inhibit or induce metabolic enzymes can alter the kinetic order of other drugs, impacting their efficacy and safety.
-
Monitoring Drug Levels: Therapeutic drug monitoring (TDM) is often used to ensure that drug levels remain within the therapeutic range. TDM is especially important for drugs that exhibit zero-order kinetics, as these drugs are more susceptible to accumulation and toxicity.
Examples of Drugs Exhibiting Different Kinetic Orders
First-Order Kinetics: Most drugs exhibit first-order kinetics. Examples include many commonly prescribed medications like:
- Paracetamol (Acetaminophen): At therapeutic doses, paracetamol is primarily eliminated through first-order kinetics.
- Aspirin: Similarly, aspirin metabolism generally follows first-order kinetics within the therapeutic range.
- Many antibiotics: Many antibiotics show first-order kinetics in their elimination.
Zero-Order Kinetics: Fewer drugs exhibit zero-order kinetics at therapeutic doses, but a few notable examples include:
- Ethanol (Alcohol): Ethanol elimination follows zero-order kinetics because the enzymes involved in its metabolism (alcohol dehydrogenase) become saturated at relatively low blood alcohol concentrations. This is why the rate of alcohol removal is relatively constant regardless of the concentration (within a certain range).
- Phenytoin: At high doses, phenytoin metabolism can switch to zero-order kinetics due to enzyme saturation.
- Salicylic acid (high doses): At high doses, salicylic acid (the metabolite of aspirin) can exhibit zero-order elimination.
Half-Life and its Significance
The half-life (t₁/₂) of a drug is the time it takes for the drug concentration to decrease by half. The concept of half-life is crucial in understanding drug kinetics and designing appropriate dosage regimens.
First-Order Kinetics: The half-life for a drug following first-order kinetics is constant and independent of the initial drug concentration. It's calculated as: t₁/₂ = 0.693/k
This means that regardless of the starting concentration, the same fraction of the drug will be eliminated within each half-life interval.
Zero-Order Kinetics: The half-life for a drug following zero-order kinetics is not constant; it depends on the initial drug concentration. The half-life equation is more complex and involves the zero-order rate constant and initial concentration. It's not as straightforward to calculate as in first-order kinetics.
The implications are that, for first-order processes, repeated dosing leads to a predictable accumulation towards a steady-state concentration. For zero-order processes, the accumulation is less predictable and more susceptible to toxicity.
Clinical Significance and Future Directions
Accurate characterization of the kinetic order of drug elimination is paramount for optimizing drug therapy. Misunderstanding the kinetic order can lead to subtherapeutic drug levels (ineffective treatment) or, more seriously, to drug toxicity. Pharmacokinetic studies using sophisticated modeling techniques are crucial in defining the kinetic order, especially for new drug candidates.
Further research continues to investigate the complexities of drug metabolism and elimination. Factors like genetic polymorphisms influencing enzyme activity and drug-drug interactions are active areas of investigation. Advances in pharmacogenomics, the study of how genes affect a person's response to drugs, are refining our understanding of individual variability in drug kinetics and tailoring treatment to individual patients' needs. Personalized medicine and precision drug development will rely heavily on our continuing progress in understanding these complex processes.
In conclusion, while first-order kinetics is the most common pattern of drug elimination, understanding the unique characteristics of zero-order kinetics is crucial for safe and effective drug therapy. The differences in elimination rate, half-life, and dose-response relationships between these two kinetic orders highlight the importance of considering the pharmacokinetic properties of a drug when designing and administering treatment. Continual advancements in our understanding of drug metabolism and elimination, aided by sophisticated modeling and pharmacogenomic approaches, will undoubtedly lead to more precise and personalized therapeutic strategies in the future.
Latest Posts
Latest Posts
-
Differentiate Between Isometric And Isotonic Contractions
Mar 30, 2025
-
Match Each Equation With A Graph Above
Mar 30, 2025
-
How Do Organisms Generate Energy When Oxygen Is Not Available
Mar 30, 2025
-
What Does Lda Do In A Reaction
Mar 30, 2025
-
What Is Found In Animal Cells But Not Plant
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about First Order Versus Zero Order Kinetics . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
