For A Review Of How To Make Alkyl Tosylates
Muz Play
Apr 07, 2025 · 5 min read
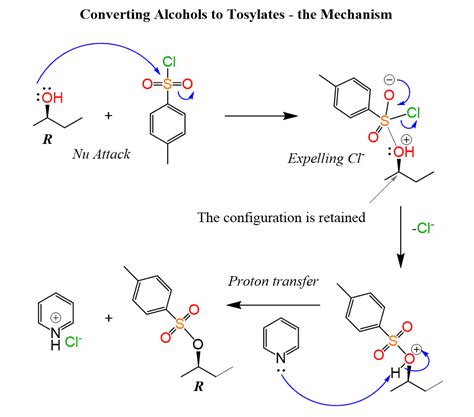
Table of Contents
A Comprehensive Review of Alkyl Tosylate Synthesis: Methods, Mechanisms, and Applications
Alkyl tosylates, also known as alkyl p-toluenesulfonates, are valuable intermediates in organic synthesis. Their versatility stems from the excellent leaving group ability of the tosylate group (-OTs), making them highly reactive in nucleophilic substitution reactions, eliminations, and other transformations. This review delves into the various methods for synthesizing alkyl tosylates, exploring the underlying mechanisms and highlighting their diverse applications in organic chemistry.
Understanding the Tosylate Group
Before diving into the synthesis methods, it's crucial to understand the nature of the tosylate group. The tosylate anion (TsO⁻) is a resonance-stabilized conjugate base of p-toluenesulfonic acid (TsOH). This resonance stabilization significantly enhances its leaving group ability, making it superior to many other leaving groups such as halides. The bulky nature of the tosylate group also plays a role in influencing reaction stereochemistry, often favoring SN2 reactions.
Resonance Stabilization of the Tosylate Anion
The negative charge on the tosylate anion is delocalized over the sulfonyl group and the aromatic ring through resonance. This delocalization significantly reduces the charge density, making it a more stable and better leaving group compared to, for example, a simple alkoxide. This stability is key to the efficacy of tosylates in various organic reactions.
Synthesis Methods for Alkyl Tosylates
The most common method for synthesizing alkyl tosylates is via the reaction of an alcohol with p-toluenesulfonyl chloride (TsCl) in the presence of a base. Several variations exist, each offering advantages depending on the substrate and desired outcome.
Method 1: Reaction with p-Toluenesulfonyl Chloride (TsCl)
This is the most prevalent method. The reaction proceeds through an SN2 mechanism, with the tosylate group replacing the hydroxyl group of the alcohol.
Reaction: ROH + TsCl → ROTs + HCl
Mechanism:
- Nucleophilic attack: The alcohol's oxygen atom acts as a nucleophile, attacking the sulfur atom of TsCl.
- Proton transfer: A proton is transferred from the alcohol oxygen to the chloride ion.
- Leaving group departure: The chloride ion departs, leading to the formation of the alkyl tosylate.
Reagents and Conditions:
- TsCl: p-Toluenesulfonyl chloride is commercially available and readily reacts with alcohols.
- Base: A base is necessary to neutralize the HCl produced during the reaction and to facilitate the departure of the chloride ion. Common bases include pyridine, triethylamine (Et3N), and 4-dimethylaminopyridine (DMAP). The choice of base depends on the sensitivity of the substrate to strong bases. Pyridine is often preferred for its mildness and its ability to act as both a base and a solvent.
- Solvent: A variety of solvents can be used, depending on the solubility of the reactants and products. Common solvents include dichloromethane (DCM), diethyl ether, and pyridine itself.
Limitations: This method is generally suitable for primary and secondary alcohols. Tertiary alcohols often undergo elimination reactions instead of substitution, leading to the formation of alkenes.
Method 2: Using Tosyl Imidazole
Tosyl imidazole is a milder alternative to TsCl. It reacts with alcohols under less acidic conditions, making it suitable for sensitive substrates.
Reaction: ROH + TsIm → ROTs + ImH
Advantages: Milder reaction conditions, reduced risk of side reactions, especially for acid-sensitive substrates.
Limitations: Tosyl imidazole is less readily available than TsCl.
Method 3: In situ Generation of TsCl
In some cases, p-toluenesulfonyl chloride can be generated in situ by reacting p-toluenesulfonic acid with thionyl chloride (SOCl2). This method is advantageous when dealing with very sensitive alcohols.
Reaction: TsOH + SOCl2 → TsCl + SO2 + HCl
Optimizing the Synthesis: Reaction Conditions and Workup
Several factors influence the yield and selectivity of alkyl tosylate synthesis:
- Temperature: Lower temperatures are generally preferred to minimize side reactions.
- Stoichiometry: A slight excess of TsCl is often used to ensure complete conversion.
- Reaction time: Sufficient reaction time is crucial for complete conversion, but prolonged reaction times may lead to side reactions.
- Workup: The reaction mixture typically requires workup to isolate the alkyl tosylate. This usually involves washing with aqueous acid to remove excess base, followed by extraction with an organic solvent and drying over an anhydrous salt.
Applications of Alkyl Tosylates
Alkyl tosylates find extensive use in various organic transformations:
1. Nucleophilic Substitution Reactions (SN1 and SN2)
The excellent leaving group ability of the tosylate makes it ideal for nucleophilic substitution reactions. Primary alkyl tosylates generally undergo SN2 reactions, while secondary and tertiary alkyl tosylates can undergo both SN1 and SN2 reactions, depending on the nucleophile and reaction conditions. This versatility allows for the introduction of a wide range of functional groups.
2. Elimination Reactions
Alkyl tosylates can undergo elimination reactions to form alkenes, particularly under basic conditions. The reaction conditions (base strength and temperature) influence the selectivity of the elimination (E1 or E2).
3. Williamson Ether Synthesis
Alkyl tosylates are excellent substrates for Williamson ether synthesis, a crucial method for forming carbon-oxygen bonds. They react with alkoxides to form ethers.
4. Formation of Carbon-Carbon Bonds
Alkyl tosylates can participate in various carbon-carbon bond-forming reactions, including Grignard reactions and other organometallic couplings.
5. Synthesis of Other Functional Groups
Alkyl tosylates serve as versatile intermediates in the synthesis of various functional groups such as amines, thioethers, and nitriles.
Conclusion
Alkyl tosylates are crucial intermediates in organic synthesis, offering a powerful and versatile handle for introducing various functional groups and constructing complex molecules. The methods described in this review, coupled with careful optimization of reaction conditions, enable the efficient and selective preparation of these important building blocks. The versatility and reactivity of alkyl tosylates make them indispensable tools for synthetic chemists. Further research into milder, more sustainable, and more efficient methods for their preparation continues to be an active area of investigation within the field of organic synthesis. Understanding the mechanisms and applications of alkyl tosylate synthesis is essential for anyone engaged in organic synthesis.
Latest Posts
Latest Posts
-
The Gain Of Electrons Is Called
Apr 10, 2025
-
Strategic Positioning Allows Managers To Blank
Apr 10, 2025
-
Lu Decomposition With Partial Pivoting Example
Apr 10, 2025
-
What Solvent Is Used In Thin Layer Chromatography
Apr 10, 2025
-
What Are Two Types Of Groups In Determining Social Categorization
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about For A Review Of How To Make Alkyl Tosylates . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.