For An Exothermic Reaction The Products
Muz Play
Mar 17, 2025 · 7 min read
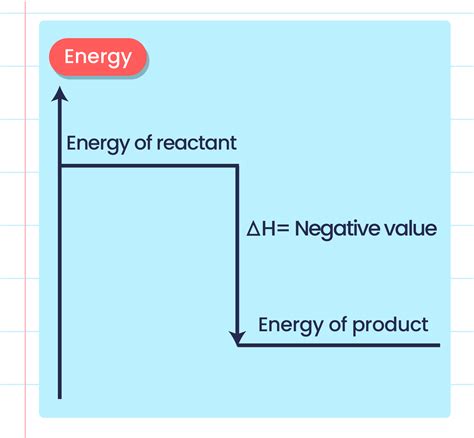
Table of Contents
For an Exothermic Reaction, the Products Have Lower Energy Than the Reactants
Exothermic reactions are a fundamental concept in chemistry, and understanding their characteristics is crucial for various applications, from industrial processes to everyday life. This article delves deep into the energy changes involved in exothermic reactions, specifically focusing on the energy state of the products compared to the reactants. We'll explore the underlying principles, provide illustrative examples, and discuss the practical implications of these reactions.
Understanding Exothermic Reactions: A Foundation
An exothermic reaction is a chemical or physical process that releases energy into its surroundings. This release of energy is usually in the form of heat, but it can also manifest as light or sound. The key defining characteristic is that the products of an exothermic reaction have lower total energy than the reactants. This energy difference is released during the reaction, resulting in a net decrease in the system's internal energy.
The Energy Profile: Visualizing the Energy Change
The energy change in a chemical reaction can be visually represented using an energy profile diagram. This diagram illustrates the potential energy of the reactants and products throughout the reaction process. For an exothermic reaction, the energy profile shows that the products are at a lower energy level than the reactants. The difference between these energy levels represents the energy released during the reaction, often referred to as the enthalpy change (ΔH). A negative ΔH value always indicates an exothermic reaction.
Key Characteristics of Exothermic Reactions:
- Heat Release: The most prominent feature is the release of heat into the surroundings. This often results in an increase in the temperature of the surroundings.
- Negative Enthalpy Change (ΔH < 0): This is a quantitative measure of the energy released. The larger the negative value, the greater the amount of energy released.
- Spontaneous Reactions (Often): While not always the case, many exothermic reactions are spontaneous under standard conditions, meaning they proceed without external intervention. However, spontaneity also depends on entropy changes.
- Stable Products: The products formed in an exothermic reaction are generally more stable than the reactants because they are at a lower energy level. This increased stability is a consequence of stronger bonds formed in the products.
Examples of Exothermic Reactions: From Everyday Life to Industrial Processes
Exothermic reactions are prevalent in various aspects of our lives and industrial processes. Here are some notable examples:
1. Combustion Reactions:
Combustion is a quintessential exothermic reaction. The burning of fuels like wood, natural gas (methane), propane, and gasoline all involve the rapid oxidation of a fuel source, releasing substantial amounts of heat and light.
Example: The combustion of methane:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) + Heat
This reaction releases a significant amount of heat, making it suitable for heating homes and powering engines.
2. Neutralization Reactions:
Acid-base neutralization reactions are also exothermic. When an acid reacts with a base, the resulting salt and water formation release heat.
Example: The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l) + Heat
This reaction is often used in calorimetry experiments to measure the heat of neutralization.
3. Respiration in Living Organisms:
Cellular respiration, the process by which organisms convert glucose into energy (ATP), is a complex series of exothermic reactions. These reactions release energy gradually, preventing cellular damage from sudden heat release.
Simplified Example: The overall reaction of glucose oxidation:
C₆H₁₂O₆(s) + 6O₂(g) → 6CO₂(g) + 6H₂O(l) + Energy (ATP)
This energy is essential for life processes.
4. Nuclear Reactions:
Nuclear fission and fusion reactions are highly exothermic, releasing immense amounts of energy. Nuclear power plants harness the energy from nuclear fission, while the sun's energy is generated through nuclear fusion. These reactions involve changes in the nucleus of atoms, leading to massive energy release.
5. Formation of Many Chemical Bonds:
The formation of many chemical bonds is inherently exothermic. When atoms combine to form molecules, energy is released as the atoms achieve lower energy states by forming stronger bonds. This energy release contributes to the overall exothermicity of a reaction.
Practical Applications and Implications:
The heat released in exothermic reactions has numerous practical applications:
- Energy Production: Combustion of fuels is the primary method for generating electricity in power plants.
- Heating and Cooling: Exothermic reactions are used in various heating systems, from home furnaces to industrial processes. Conversely, the controlled absorption of heat (endothermic processes) can be used for cooling.
- Industrial Processes: Many industrial chemical processes, such as the production of ammonia (Haber-Bosch process), rely on exothermic reactions.
- Chemical Synthesis: Exothermic reactions are often utilized in the synthesis of new compounds, as the released energy can drive the reaction forward.
Understanding the Energy States: Bond Energies and Enthalpy
To fully appreciate why products of exothermic reactions have lower energy, we need to examine bond energies and enthalpy.
Bond Energies:
Bond energy refers to the energy required to break a chemical bond. In exothermic reactions, the total bond energy of the reactants is higher than the total bond energy of the products. This means that more energy is released when new bonds are formed in the products than is required to break the bonds in the reactants. This difference in bond energies is reflected in the negative ΔH value.
Enthalpy Change (ΔH):
Enthalpy is a thermodynamic property that represents the total heat content of a system at constant pressure. The enthalpy change (ΔH) is the difference in enthalpy between the products and reactants. For exothermic reactions, ΔH is negative, indicating a net release of heat to the surroundings.
The enthalpy change can be calculated using various methods, including:
- Using standard enthalpies of formation: This involves using tabulated values of the standard enthalpies of formation for the reactants and products.
- Using Hess's Law: This allows the calculation of ΔH for a reaction by summing the ΔH values for a series of reactions that add up to the overall reaction.
- Experimental measurement using calorimetry: This involves measuring the heat released or absorbed during a reaction using a calorimeter.
Factors Affecting Exothermic Reaction Rates:
Several factors influence the rate at which an exothermic reaction proceeds:
- Temperature: Increasing the temperature generally increases the reaction rate. This is because higher temperatures provide more kinetic energy to the reactant molecules, leading to more frequent and energetic collisions.
- Concentration: Higher concentrations of reactants lead to more frequent collisions, thus increasing the reaction rate.
- Surface Area: For reactions involving solids, increasing the surface area of the solid reactant increases the rate, as more reactant molecules are exposed to the other reactants.
- Presence of a Catalyst: Catalysts speed up reactions without being consumed themselves. They provide an alternative reaction pathway with a lower activation energy, making it easier for the reaction to occur.
Exothermic Reactions and Entropy: A Deeper Dive
While exothermic reactions often appear spontaneous, spontaneity is governed by both enthalpy and entropy. Entropy (S) is a measure of disorder or randomness in a system. The change in entropy (ΔS) during a reaction also contributes to the overall spontaneity. The Gibbs free energy (ΔG) combines both enthalpy and entropy to determine spontaneity:
ΔG = ΔH - TΔS
- If ΔG < 0: The reaction is spontaneous.
- If ΔG > 0: The reaction is non-spontaneous.
- If ΔG = 0: The reaction is at equilibrium.
Even a highly exothermic reaction (negative ΔH) can be non-spontaneous if the decrease in entropy (negative ΔS) is large enough at a given temperature. Conversely, a reaction that is slightly endothermic but has a large increase in entropy can be spontaneous.
Conclusion: The Significance of Exothermic Reactions
Exothermic reactions play a vital role in various aspects of our lives and industrial processes. Understanding the energy changes involved, the energy states of the products relative to the reactants, and the factors affecting their rates is crucial for a comprehensive understanding of chemistry and its applications. From the burning of fuels to the processes within living organisms, the principles of exothermic reactions are fundamental to our world. Further research continues to unveil new applications and refine our understanding of these vital processes. The release of energy in exothermic reactions remains a powerful force shaping our environment and driving countless processes, both natural and synthetic.
Latest Posts
Latest Posts
-
Name A Structural Difference Between Triglycerides And Phospholipids
Mar 17, 2025
-
Ending Materials In A Chemical Reaction
Mar 17, 2025
-
Get Energy By Eating Other Organisms
Mar 17, 2025
-
What Is A Power Function In Math
Mar 17, 2025
-
How Many Phosphate Groups Does Atp Have
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about For An Exothermic Reaction The Products . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
