Function Of Salt Bridge In Voltaic Cell
Muz Play
Mar 18, 2025 · 6 min read
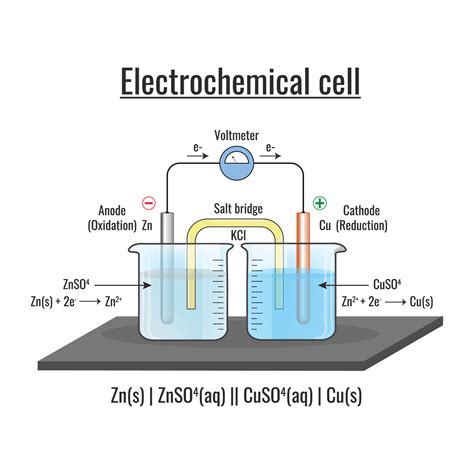
Table of Contents
The Crucial Role of the Salt Bridge in Voltaic Cells: Ensuring Electrical Neutrality and Continuous Current Flow
Voltaic cells, also known as galvanic cells, are electrochemical devices that convert chemical energy into electrical energy. They are the powerhouses behind many everyday applications, from batteries in our electronic devices to powering vehicles. A key component ensuring the smooth operation of these cells is the salt bridge. This seemingly simple component plays a critical role in maintaining the cell's functionality and preventing it from quickly becoming inoperable. This article will delve into the intricate function of the salt bridge, exploring its necessity, the consequences of its absence, and the various types available.
Understanding the Fundamentals of Voltaic Cells
Before we delve into the specifics of the salt bridge, it's essential to grasp the basic principles governing voltaic cells. These cells consist of two half-cells, each containing an electrode immersed in an electrolyte solution. One half-cell is the anode, where oxidation (loss of electrons) occurs, and the other is the cathode, where reduction (gain of electrons) occurs. The electrons released at the anode flow through an external circuit, powering the device connected to the cell, before reaching the cathode.
This electron flow creates an electric current. However, the process isn't as straightforward as it seems. If the electron flow were the only factor, the reaction would quickly cease. This is because the buildup of positive charge at the anode and negative charge at the cathode would create an opposing potential, halting the flow of electrons. This is where the salt bridge steps in, acting as a vital mediator.
The Salt Bridge: Maintaining Electrical Neutrality
The primary function of the salt bridge is to maintain electrical neutrality within the two half-cells. As oxidation occurs at the anode, positive ions accumulate in the solution. Simultaneously, reduction at the cathode leads to an increase in negative ions. Without a salt bridge, these charge imbalances would rapidly build up, creating an opposing potential difference that counteracts the cell potential and stops the electron flow.
The salt bridge allows for the migration of ions between the two half-cells. It contains an electrolyte, typically a saturated solution of an inert salt like potassium nitrate (KNO₃) or potassium chloride (KCl), which provides mobile ions. These ions are chosen for their inertness; they shouldn't participate in the redox reactions occurring in the half-cells.
Ion Migration: The Balancing Act
As positive ions accumulate at the anode, negative ions from the salt bridge migrate into the anode compartment to neutralize the excess positive charge. Conversely, positive ions from the salt bridge move into the cathode compartment to balance the excess negative charge. This migration of ions ensures that the charge remains balanced in both half-cells, allowing the redox reactions to continue and the current to flow uninterrupted.
Consequences of the Absence of a Salt Bridge
Without a salt bridge, a voltaic cell would quickly become inoperable. The charge buildup in the half-cells would immediately create a potential difference opposing the cell's potential. This opposing potential would halt the electron flow, effectively stopping the chemical reaction and electricity generation. The cell would be "dead" almost instantly.
Types of Salt Bridges and Their Characteristics
While saturated solutions of potassium nitrate or potassium chloride are common, several types of salt bridges exist, each with its own advantages and disadvantages:
-
U-shaped tube filled with electrolyte: This is the most common type, visually representing a simple U-shaped tube filled with an electrolyte solution. Its simplicity and effectiveness make it ideal for many applications.
-
Agar-agar gel electrolyte: This type uses agar-agar, a gelatinous substance derived from seaweed, to immobilize the electrolyte solution within the salt bridge. This design prevents the mixing of solutions but might have slightly higher electrical resistance.
-
Filter paper soaked in electrolyte: This is a simpler, cheaper alternative, using filter paper soaked in the electrolyte solution. It's less durable and may be less effective than the other types.
-
Porous membrane: A porous membrane acts as a physical barrier while still permitting ion transport, separating the two compartments without the need for a separate salt bridge. This offers a compact and integrated design.
Optimizing Salt Bridge Performance
The performance of a salt bridge can impact the overall efficiency of the voltaic cell. Several factors influence this performance:
-
Electrolyte Concentration: A higher concentration of the electrolyte generally leads to faster ion migration and improved cell performance. However, excessively high concentrations may lead to unwanted side reactions.
-
Electrolyte Type: The choice of electrolyte is crucial. It must be chemically inert and possess sufficient ionic conductivity. Potassium nitrate and potassium chloride are frequently used due to their high solubility and low reactivity.
-
Salt Bridge Length and Diameter: The length and diameter of the salt bridge affect the resistance to ion flow. Shorter and wider bridges reduce resistance, allowing for faster ion migration and better cell performance. However, extremely short bridges may increase the likelihood of solution mixing.
-
Temperature: Higher temperatures typically lead to increased ionic mobility and thus enhanced salt bridge performance.
The Salt Bridge and its Role in Specific Cell Types
The function of the salt bridge remains consistent across different types of voltaic cells. Whether it's a simple Daniell cell, a more complex fuel cell, or even specialized electrochemical systems, the salt bridge maintains electrical neutrality, enabling continued electron flow.
Consider, for example, a Daniell cell. This simple cell uses zinc and copper electrodes. Without the salt bridge, the accumulation of positive zinc ions at the anode and negative sulfate ions at the cathode would rapidly halt the reaction. The salt bridge ensures that the solution remains electrically neutral.
Practical Applications and Beyond
Understanding the function of the salt bridge extends beyond simple classroom demonstrations. It’s essential for:
-
Battery Design: Optimizing salt bridge design is vital in enhancing battery performance, extending battery life, and increasing their power output.
-
Electroplating: The principle applies to electroplating, where controlled ion movement is critical for depositing a uniform metal layer.
-
Corrosion Control: Salt bridges are relevant in studying and mitigating corrosion, where electrochemical processes contribute to material degradation.
-
Electrochemical Sensors: Salt bridges maintain the ionic balance in electrochemical sensors, ensuring reliable and accurate measurements.
Conclusion: An Unsung Hero of Electrochemical Devices
The salt bridge is an often-overlooked yet indispensable component in voltaic cells. Its seemingly simple function – maintaining electrical neutrality – is crucial for the continued operation of these devices. Without it, the electrochemical reactions would quickly cease, rendering the cell useless. Understanding the various types of salt bridges, their characteristics, and the factors affecting their performance is essential for optimizing the efficiency and longevity of voltaic cells and numerous other electrochemical applications. The seemingly simple salt bridge is, in fact, a critical element that ensures the success of countless electrochemical processes.
Latest Posts
Latest Posts
-
Convert The Following Complex Number Into Its Polar Representation
Mar 19, 2025
-
What Elements Are Contained In Proteins
Mar 19, 2025
-
Draw The Lewis Dot Diagram For A Cation
Mar 19, 2025
-
Iron Rusting Chemical Or Physical Change
Mar 19, 2025
-
Under Acid Hydrolysis Conditions Starch Is Converted To
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Function Of Salt Bridge In Voltaic Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
