Gas To Liquid Endothermic Or Exothermic
Muz Play
Mar 30, 2025 · 6 min read
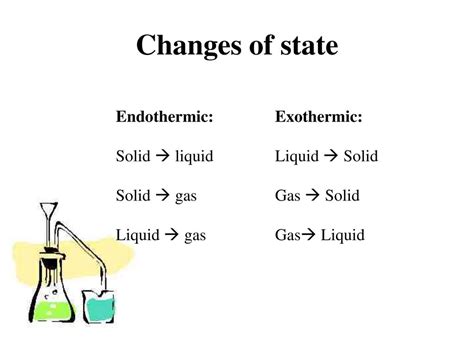
Table of Contents
Gas to Liquid Conversion: Understanding Endothermic and Exothermic Reactions
The conversion of gas to liquid is a crucial process in various industries, from natural gas processing to the production of biofuels. Understanding the thermodynamics of this conversion, specifically whether it's an endothermic or exothermic reaction, is essential for optimizing efficiency and controlling the process. This article will delve deep into the complexities of gas-to-liquid (GTL) processes, clarifying the endothermic and exothermic aspects involved, examining different conversion methods, and exploring the factors influencing the overall energy balance.
What are Endothermic and Exothermic Reactions?
Before diving into the specifics of GTL, let's establish a clear understanding of the fundamental thermodynamic concepts.
Endothermic reactions absorb energy from their surroundings. This energy absorption is typically manifested as a decrease in temperature of the reaction environment. The products of an endothermic reaction possess more energy than the reactants. Think of it like charging a battery – energy needs to be inputted to store it.
Exothermic reactions, on the other hand, release energy to their surroundings. This energy release often appears as an increase in temperature. The products of an exothermic reaction possess less energy than the reactants. This is analogous to discharging a battery – energy is released as it's used.
Gas to Liquid Conversion Methods: A Spectrum of Reactions
Several methods exist for converting gases into liquids, each with its own unique thermodynamic characteristics. The overall energy balance – whether the process is predominantly endothermic or exothermic – depends heavily on the specific method employed and the operating conditions.
1. Fischer-Tropsch Process: A Complex Mixture
The Fischer-Tropsch process is a prominent example of GTL technology. It involves the conversion of synthesis gas (syngas), a mixture of carbon monoxide (CO) and hydrogen (H₂), into liquid hydrocarbons. This process is generally exothermic, meaning it releases heat. However, the overall energy balance can be influenced by several factors:
-
Reaction Conditions: Temperature and pressure significantly impact the reaction's exothermicity. Higher temperatures can lead to a decrease in the overall heat release.
-
Catalyst Type: Different catalysts can affect the reaction kinetics and heat generation. Optimizing catalyst selection is crucial for maximizing efficiency and controlling the reaction's heat output.
-
Product Distribution: The types and quantities of liquid hydrocarbons produced influence the heat balance. The formation of heavier hydrocarbons tends to release more heat than the formation of lighter ones.
The exothermic nature of the Fischer-Tropsch process allows for the recovery of heat, which can be used to improve process efficiency and reduce energy costs. However, effective heat management is crucial to avoid overheating and potential catalyst deactivation.
2. Methanol Synthesis: An Exothermic Pathway to Liquid Fuel
Methanol synthesis, another crucial GTL process, involves converting syngas into methanol (CH₃OH). This reaction is also exothermic. The heat released can be harnessed to generate steam or electricity, contributing to a more efficient and sustainable operation. Similar to the Fischer-Tropsch process, the specific reaction conditions, catalyst type, and product yield affect the degree of exothermicity.
3. Direct Liquefaction of Coal and Biomass: A Diverse Energy Landscape
The direct liquefaction of coal and biomass offers an alternative route to producing liquid fuels from gaseous precursors. These processes are characterized by a complex interplay of endothermic and exothermic reactions.
-
Coal Liquefaction: Involves several steps, including pyrolysis (an endothermic decomposition process) and hydrogenation (often exothermic). The net energy balance can vary widely depending on the coal type, process parameters, and the specific reaction pathways involved.
-
Biomass Liquefaction: Similar to coal liquefaction, it involves a combination of endothermic and exothermic steps. The overall energy balance depends heavily on the biomass feedstock and the process conditions.
The complexity of these processes necessitates careful optimization of reaction conditions to maximize liquid fuel yield and minimize energy consumption.
4. Other GTL Technologies: Exploring the Possibilities
Numerous other GTL technologies are under development, each presenting its unique thermodynamic profile. Some processes may involve predominantly endothermic reactions, such as certain types of gas hydrates formation under specific conditions. Others might exhibit a more balanced combination of endothermic and exothermic steps.
Factors Influencing the Endothermic/Exothermic Nature of GTL Processes
Several crucial factors contribute to the overall energy balance in gas-to-liquid conversion processes:
-
Reaction Temperature and Pressure: These parameters have a profound effect on the reaction kinetics and equilibrium. Altering these parameters can shift the balance between endothermic and exothermic reactions.
-
Catalyst Selection: The type of catalyst employed significantly influences the reaction pathway and the associated energy changes. Catalyst optimization is a critical factor in achieving high efficiency and controlling the overall heat balance.
-
Gas Composition: The composition of the feed gas (e.g., syngas composition, presence of impurities) directly affects the reaction thermodynamics.
-
Product Distribution: The types and quantities of liquid products formed have a significant impact on the heat released or absorbed.
Industrial Applications and Implications
The understanding of the endothermic and exothermic nature of GTL processes is crucial for various industrial applications:
-
Energy Efficiency: In exothermic processes, the released heat can be recovered and utilized, enhancing overall energy efficiency. In endothermic processes, careful consideration of energy input is necessary.
-
Process Optimization: Optimizing reaction conditions, catalyst selection, and reactor design is essential for maximizing the efficiency of both exothermic and endothermic processes.
-
Environmental Impact: The energy balance of GTL processes directly impacts their overall environmental footprint. Processes with high energy efficiency contribute to reduced greenhouse gas emissions.
-
Economic Viability: The cost-effectiveness of GTL technologies depends heavily on the energy efficiency and the overall operational costs.
Future Directions: Innovation and Sustainability in GTL
Ongoing research and development efforts focus on improving the efficiency and sustainability of GTL processes. This includes:
-
Developing more efficient and selective catalysts: This can lead to improved conversion rates and reduced energy consumption.
-
Optimizing reactor design: Advanced reactor designs can enhance heat transfer and improve overall process efficiency.
-
Integrating renewable energy sources: Using renewable energy sources (e.g., solar, wind) to power endothermic GTL processes can significantly reduce their carbon footprint.
-
Exploring new GTL technologies: Research continues into novel methods for gas-to-liquid conversion, potentially leading to more efficient and sustainable processes.
Conclusion: A Dynamic Field of Research and Innovation
The conversion of gas to liquid is a complex process involving a wide spectrum of endothermic and exothermic reactions. A deep understanding of these thermodynamic aspects is crucial for optimizing process efficiency, minimizing environmental impact, and ensuring economic viability. Continued research and development efforts are crucial for advancing GTL technologies, paving the way for more sustainable and cost-effective production of liquid fuels and chemicals. The field is dynamic, with ongoing innovations promising improvements in efficiency and sustainability, highlighting the importance of continuous learning and adaptation in this ever-evolving technological landscape. Further research into novel catalysts and process optimization strategies will continue to shape the future of GTL, potentially revolutionizing energy production and chemical manufacturing.
Latest Posts
Latest Posts
-
The Envelope Of A Virus Is Derived From The Hosts
Apr 01, 2025
-
Plant Cell In A Hypotonic Solution
Apr 01, 2025
-
What Is The Definition Of Form In Music
Apr 01, 2025
-
Staph Epidermidis Hemolysis On Blood Agar
Apr 01, 2025
-
Difference Between Autonomic And Somatic Nervous System
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Gas To Liquid Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
