General Form Of A Rate Law
Muz Play
Mar 16, 2025 · 6 min read
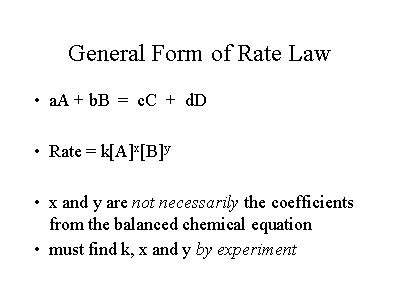
Table of Contents
- General Form Of A Rate Law
- Table of Contents
- The General Form of a Rate Law: Understanding Reaction Kinetics
- Understanding the Fundamentals: Rate, Reactants, and Products
- The General Form of a Rate Law
- Deciphering the Rate Constant (k)
- Understanding Reaction Orders (m, n, ...)
- Determining the Rate Law Experimentally
- Complex Rate Laws and Reaction Mechanisms
- The Significance of Rate Laws in Chemistry and Beyond
- Advanced Concepts and Applications
- Conclusion: Mastering the General Form of a Rate Law
- Latest Posts
- Latest Posts
- Related Post
The General Form of a Rate Law: Understanding Reaction Kinetics
Chemical kinetics is the study of reaction rates, exploring how quickly reactants transform into products. A cornerstone of this field is the rate law, a mathematical expression that describes the relationship between the rate of a reaction and the concentrations of the reactants. Understanding the general form of a rate law is crucial for predicting reaction behavior, designing efficient processes, and deciphering reaction mechanisms. This comprehensive guide will delve into the intricacies of rate laws, exploring their general form, the significance of rate constants and reaction orders, and the methods used to determine them experimentally.
Understanding the Fundamentals: Rate, Reactants, and Products
Before diving into the general form, let's establish a firm understanding of the key components:
-
Reaction Rate: This refers to the speed at which a reaction proceeds, typically expressed as the change in concentration of a reactant or product per unit time. It's often measured in units like mol L⁻¹ s⁻¹. The rate can vary depending on factors like temperature, concentration, and the presence of catalysts.
-
Reactants: These are the substances consumed during a chemical reaction. Their concentrations directly influence the rate of the reaction.
-
Products: These are the substances formed as a result of the chemical reaction.
The General Form of a Rate Law
The general form of a rate law for a reaction can be expressed as:
Rate = k[A]<sup>m</sup>[B]<sup>n</sup>...
Where:
- Rate: Represents the rate of the reaction.
- k: Is the rate constant, a proportionality constant specific to the reaction at a given temperature. It reflects the intrinsic speed of the reaction.
- [A], [B], ...: Represent the molar concentrations of reactants A, B, and so on.
- m, n, ...: Represent the reaction orders with respect to reactants A, B, and so on. These are exponents determined experimentally and are not necessarily equal to the stoichiometric coefficients in the balanced chemical equation.
Deciphering the Rate Constant (k)
The rate constant, k, is a crucial parameter in the rate law. It quantifies the intrinsic reactivity of the system at a particular temperature. A higher value of k indicates a faster reaction. The temperature dependence of k is typically described by the Arrhenius equation:
k = Ae<sup>-Ea/RT</sup>
Where:
- A: Is the pre-exponential factor or frequency factor, representing the frequency of collisions between reactant molecules.
- Ea: Is the activation energy, the minimum energy required for a reaction to occur.
- R: Is the ideal gas constant.
- T: Is the absolute temperature.
The Arrhenius equation reveals that the rate constant, and therefore the reaction rate, increases exponentially with temperature. This is because higher temperatures lead to more frequent and energetic collisions between reactant molecules, increasing the likelihood of successful reactions.
Understanding Reaction Orders (m, n, ...)
The reaction orders (m, n, etc.) are exponents in the rate law that indicate the sensitivity of the reaction rate to changes in the concentration of each reactant. These orders are experimentally determined and can be integers, fractions, or even zero.
-
Zero-Order Reaction: The rate is independent of the concentration of the reactant (m = 0). The rate remains constant regardless of the reactant concentration.
-
First-Order Reaction: The rate is directly proportional to the concentration of the reactant (m = 1). Doubling the concentration doubles the rate.
-
Second-Order Reaction: The rate is proportional to the square of the concentration of the reactant (m = 2). Doubling the concentration quadruples the rate.
It's important to note that the overall reaction order is the sum of the individual reaction orders (m + n + ...). For example, a reaction with a rate law of Rate = k[A][B]² is first-order with respect to A, second-order with respect to B, and third-order overall.
Determining the Rate Law Experimentally
The rate law cannot be determined solely from the balanced chemical equation. Experimental data is crucial. Common methods include:
-
Method of Initial Rates: This involves measuring the initial rate of the reaction at different initial concentrations of reactants. By comparing the rates at different concentrations, the reaction orders can be determined.
-
Integrated Rate Laws: These are mathematical expressions derived from the rate law that relate the concentration of a reactant to time. By plotting the appropriate data, the reaction order and rate constant can be determined. For example, a first-order reaction yields a linear plot of ln[A] versus time, while a second-order reaction yields a linear plot of 1/[A] versus time.
Complex Rate Laws and Reaction Mechanisms
While the general form presented above applies to many reactions, some reactions exhibit more complex rate laws. These complexities often arise from multi-step reaction mechanisms. A reaction mechanism is a series of elementary steps that describe the actual pathway of the reaction at a molecular level. The overall rate law is determined by the slowest step in the mechanism, known as the rate-determining step.
For instance, a reaction might involve several intermediate species that are formed and consumed during the process. These intermediates do not appear in the overall stoichiometric equation but can significantly influence the rate law. In such cases, the rate law may involve concentrations of intermediates or display fractional reaction orders.
The Significance of Rate Laws in Chemistry and Beyond
Understanding rate laws is fundamental to various fields within chemistry and beyond:
-
Chemical Engineering: Designing efficient chemical reactors and optimizing reaction conditions require a thorough understanding of reaction kinetics and rate laws.
-
Environmental Chemistry: Predicting the fate and transport of pollutants in the environment often relies on knowledge of reaction rates and the factors that influence them.
-
Biochemistry: Enzyme kinetics, the study of enzyme-catalyzed reactions, heavily utilizes rate laws to understand enzyme activity and regulation.
-
Materials Science: The synthesis and characterization of new materials often involve reactions with specific rate requirements and kinetic considerations.
Advanced Concepts and Applications
The study of reaction kinetics and rate laws extends beyond the fundamental concepts discussed above. Several advanced topics warrant further investigation:
-
Catalysis: Catalysts accelerate reaction rates by providing alternative reaction pathways with lower activation energies. Understanding how catalysts affect the rate law is crucial in catalyst design and application.
-
Photochemistry: Light-induced reactions have their own unique kinetic features, often involving excited states and photochemical rate constants.
-
Electrochemistry: Electrochemical reactions involve electron transfer processes and their kinetics are described using specific rate laws incorporating electrode potentials and current densities.
Conclusion: Mastering the General Form of a Rate Law
The general form of a rate law, Rate = k[A]<sup>m</sup>[B]<sup>n</sup>..., is a cornerstone of chemical kinetics. Understanding its components—the rate constant (k), reaction orders (m, n, ...), and their experimental determination—is essential for predicting reaction behavior and optimizing processes across diverse chemical applications. While the basic form provides a framework, the intricacies of reaction mechanisms and more complex rate laws necessitate a deeper exploration into the rich field of reaction kinetics. By mastering these concepts, one can gain a profound understanding of the dynamics of chemical transformations and their impact on the world around us.
Latest Posts
Latest Posts
-
Conversion Of Cartesian To Cylindrical Coordinates
Mar 17, 2025
-
Which Era Is Referred To As The Age Of Mammals
Mar 17, 2025
-
How To Analyze Mass Spectrometry Data
Mar 17, 2025
-
Difference Between Synapse And Button Terminal
Mar 17, 2025
-
What Seismic Wave Travels The Fastest
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about General Form Of A Rate Law . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
