Group 17 On The Periodic Table Is Called
Muz Play
Apr 07, 2025 · 6 min read
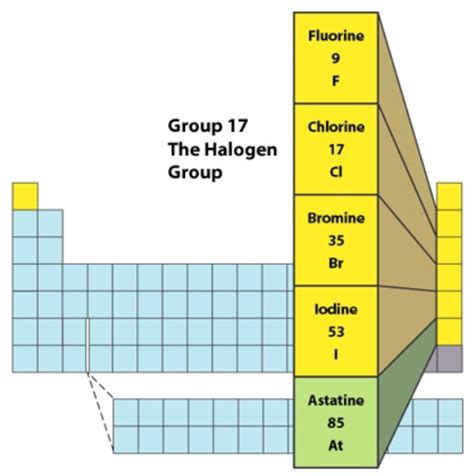
Table of Contents
Group 17 on the Periodic Table is Called the Halogens: A Deep Dive into Their Properties, Reactions, and Applications
Group 17 of the periodic table, also known as halogens, is a fascinating family of nonmetals that exhibit a unique set of properties and play crucial roles in various aspects of our lives. From essential components in everyday products to their critical roles in biological processes, understanding halogens is key to comprehending the world around us. This comprehensive article delves deep into the characteristics, reactions, and applications of this remarkable group of elements.
Understanding the Halogen Family: Key Characteristics
The halogens—fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)—are characterized by their high electronegativity and reactivity. This high reactivity stems from their electron configuration, with seven valence electrons in their outermost shell. This near-complete octet makes them highly inclined to gain one electron to achieve a stable noble gas configuration. This tendency to gain an electron is what defines their chemical behavior.
1. Electronegativity: A Driving Force
Electronegativity, the ability of an atom to attract electrons towards itself in a chemical bond, is exceptionally high for halogens. Fluorine, the most electronegative element, exhibits the strongest pull on electrons, making it exceptionally reactive. As we move down the group, electronegativity decreases, leading to a gradual reduction in reactivity.
2. Reactivity: A Defining Feature
The high reactivity of halogens is evident in their readiness to form ionic compounds with metals. They readily accept an electron from a metal atom, forming a negatively charged halide ion (e.g., F⁻, Cl⁻, Br⁻, I⁻). These ionic compounds, often called salts, are generally stable and have a wide range of applications.
3. Physical States and Properties: A Gradual Transition
Halogens exhibit a fascinating transition in their physical states as we move down the group. Fluorine and chlorine are gases at room temperature, bromine is a liquid, and iodine is a solid. This transition reflects the increasing strength of intermolecular forces with increasing atomic size and mass. Their colors also change dramatically, from the pale yellow of fluorine to the dark violet-gray of iodine.
4. Oxidation States: Versatility in Chemical Reactions
Halogens commonly exhibit a -1 oxidation state, corresponding to the gain of one electron. However, they can also exhibit positive oxidation states, particularly in compounds with more electronegative elements like oxygen. This versatility contributes to their diverse chemical behavior and allows them to participate in a wide range of reactions.
Chemical Reactions of Halogens: A Spectrum of Interactions
The reactivity of halogens is showcased in a variety of chemical reactions. Their interactions with metals, nonmetals, and other halogens provide valuable insights into their chemical behavior.
1. Reactions with Metals: Formation of Halides
Halogens readily react with metals to form metal halides. These reactions are often vigorous and exothermic, releasing significant amounts of heat. For example, the reaction of sodium with chlorine produces sodium chloride (NaCl), common table salt:
2Na(s) + Cl₂(g) → 2NaCl(s)
This reaction exemplifies the strong tendency of halogens to gain an electron and the metals to lose one. The resulting ionic bonds hold the metal and halide ions together in a crystalline lattice.
2. Reactions with Nonmetals: Covalent Compound Formation
Halogens also react with nonmetals to form covalent compounds. These compounds involve the sharing of electrons between the halogen atom and the nonmetal atom. Examples include hydrogen halides (HF, HCl, HBr, HI) and interhalogen compounds (e.g., ClF₃, BrF₅).
3. Halogen Displacement Reactions: A Hierarchy of Reactivity
Halogens exhibit a clear trend in their reactivity: fluorine is the most reactive, followed by chlorine, bromine, and iodine. This reactivity allows for halogen displacement reactions, where a more reactive halogen can displace a less reactive one from its compounds. For example, chlorine can displace bromine from a bromide salt:
Cl₂(g) + 2KBr(aq) → 2KCl(aq) + Br₂(l)
This reaction demonstrates the relative strength of the halogen-halogen bond and the tendency for the more reactive halogen to gain electrons.
4. Reactions with Water: Variable Outcomes
The reactions of halogens with water are complex and vary depending on the halogen involved. Fluorine reacts violently with water, producing oxygen and hydrofluoric acid. Chlorine, bromine, and iodine react less vigorously, forming a mixture of acids and other products.
Applications of Halogens: From Everyday Life to Specialized Uses
The unique properties of halogens translate into a broad range of applications across various fields.
1. Fluorine: Essential in Everyday Materials
Fluorine, despite its high reactivity, finds widespread use in the production of fluorocarbons. These compounds are incredibly stable and have diverse applications, including refrigerants, non-stick coatings (like Teflon), and dental products (fluoride). Fluoride also plays a critical role in preventing tooth decay.
2. Chlorine: A Versatile Chemical with Diverse Applications
Chlorine is perhaps the most versatile halogen, with applications ranging from water purification to the production of plastics (PVC). It's a powerful disinfectant, effectively killing bacteria and viruses in water treatment plants. It is also used in the production of numerous chemicals and solvents.
3. Bromine: A Crucial Component in Flame Retardants and Other Applications
Bromine is used in the production of flame retardants, which are added to various materials to reduce their flammability. It's also found in certain agricultural chemicals and photographic materials.
4. Iodine: Essential for Human Health and Industrial Processes
Iodine is essential for human health, playing a crucial role in thyroid hormone production. Iodine deficiency can lead to serious health problems, emphasizing the importance of iodine in our diet. It is also used as a disinfectant and in certain industrial processes.
Environmental Considerations and Safety Precautions
While halogens are vital for numerous applications, their use also raises environmental concerns. Certain halogenated compounds, particularly chlorofluorocarbons (CFCs), have been linked to ozone depletion. This has led to international regulations aimed at phasing out the use of ozone-depleting substances. Other halogenated compounds can also persist in the environment and accumulate in living organisms, potentially causing harmful effects.
Safety precautions are crucial when handling halogens, particularly fluorine and chlorine, due to their high reactivity. Appropriate safety equipment, including gloves, goggles, and respirators, should always be used to minimize the risk of exposure.
Astatine: The Radioactive Halogen
Astatine, the last member of the halogen family, is a radioactive element with a very short half-life. Its properties are less well-studied due to its radioactivity and scarcity. However, research on astatine continues to provide insights into the behavior of this unique element and its potential applications in nuclear medicine.
Conclusion: The Enduring Significance of Halogens
Halogens, despite their high reactivity, are essential components of various aspects of our lives. Their properties and reactions have led to numerous applications, ranging from everyday products to advanced technologies. However, responsible use and environmental considerations are crucial in minimizing potential risks associated with their applications. Continued research into halogens will undoubtedly unveil further insights into their behavior and lead to new and innovative applications. Understanding the unique characteristics of this group is vital for advancements in chemistry, medicine, and materials science. The study of halogens is an ongoing journey of scientific discovery, with new applications and understandings constantly emerging.
Latest Posts
Latest Posts
-
What Are The Formulas For Photosynthesis And Cellular Respiration
Apr 09, 2025
-
Equilibrium Constant Of Fescn2 Lab Answers
Apr 09, 2025
-
Group 7a Elements Are Also Called
Apr 09, 2025
-
Are More Electronegative Atoms More Acidic
Apr 09, 2025
-
Natural Selection And The Evolution Of Darwins Finches Answer Key
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Group 17 On The Periodic Table Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.